Primary Ovarian Insufficiency (POI) and Early/Premature Menopause: What does the hair loss specialist need to know?
Identifying Incipient Early Menopause and Primary Ovarian Insufficiency: Key Learning Points for the Hair Specialist
The average age of menopause in much of the western world is between 51 and 52 years of age. “Early menopause” is defined as menopause between age 40-45. This occurs in about 5 % of women age 40-45. “Premature menopause” is menopause that occurs before age 40. This occurs in about 1 % of women under age 40. 1 in 1000 women have menopause before age 30.
The term “primary ovarian insufficiency” (POI) refers to declining ovarian function before age 40 without a complete cessation of menstrual cycles. Women with POI may have irregular cycles but POI does not necessarily correlate with an inability to become pregnant. In fact, 5-10 % of women with POI can become pregnant naturally. The ovaries in patients with POI, however, are not producing normal amounts of hormones and are not producing follicles and eggs in the same manner as before.
In my opinion, the definition of POI really matters. POI is somewhat different than premature menoapuse. The exact definition of POI is still not agreed upon by everyone. The ‘classical’ definition had been 4 months of not having a period together with high menopausal level FSH tests (ie above 35-40 mIU/L). However, this is probably not the best definition given that many women with POI have intermittent periods. A better definition is 3 or more months or irregular periods (either no periods, long duration between periods, heavier periods) together with high FSH lab tests on two different occassions)
In 90 % of cases of POI, the cause is not known. There may be certain genetic and autoimmune links. Women with POI are at increased risk to develop autoimmune conditions like hypothyroidism, adrenal insufficiency, type 1 diabetes, pernicious anemia, myasthenia graves, connective tissue dissorders and hypothyroidism. POI was once referred to as premature ovarian ”failure” (POF) but this term is not used any more.
Early menopause and premature menopause are easier to diagnose than Primary Ovarian Insufficiency (POI) - at least if exact and precise definitions are used. This is because menopause is associated with a complete cessation of menstrual cycles for 12 months or more and POI is not. If periods have completely stopped for more than 1 year, there is a good chance the patient has menopause (assuming they are not taking hormone based medications like birth control). If the patient is 40-45 years of age, we call this early menopause and if she is under 40 we call it premature menopause. There are other reasons for periods to cease besides menopause, but this is a common reason for a cessation of periods longer than 12 months. women with POI are under 40 but don’t meet the criteria for complete cessation of periods. They have cycles intermittently.
It is a valuable skill for the hair specialist is to be able to recognize the possibility of POI and get patients connected to the right specialists who can help. POI is challenging to recognize because symptoms are not always very specific. A 33 year old female patient with fatigue, poor sleep, weight gain and changing periods may have primary ovarian insufficiency but the symptoms are not specific and most 33 year olds with fatigue, poor sleep and weight gain and changing periods will not have primary ovarian insufficiency. It’s important to know when the condition is a “possibility” even if a remote possibility. Many lives have been changed by the consideration that POI could at least be possible.
Risk Factors for Early Menopause and POI.
Several factors are known to increase the risk of early menopause and POI. The age that one enters menopause is thought to be highly inherited so the age of menopause in the patient’s mother is very very relevant. Other factors that affect the age of menopause include oophorectomy (surgery to remove the ovaries), hysterectomy (surgery to remove the uterus), smoking, chemotherapy, a family history of early menopause and chromosomal abnormalities (Turner syndrome, Fragile X). In addition. some autoimmune diseases are associated with early menopause including rheumatoid arthritis, thyroid disease and inflammatory bowel diseases. Chronic fatigue syndrome may be associated with early menopause as well. Frontal fibrosing alopecia is an autoimmune disease that is seen in the hair clinic that is also associated with early menopause.
See: FFA in Women Under 40: The Importance of Diagnosis
Signs and Symptoms of Early Menopause.
If can be challenging sometimes to recognize the very earliest signs of the menopausal transition - especially in the hair clinic when one is focused mainly on - hair loss. However, the two can be closely linked given that the hormonal changes of early menopause sometimes prompt hair loss and acceleration of an androgenetic alopecia-like picture. Also, I am always on the lookout for possible immune mediated hair disease in patients with early menopause. Frontal fibrosing alopecia as mentioned above is the main one, but lichen planopilaris should be considered.
The first clue to prompt me to consider the possibility of possible incipient early/premature menopause or primary ovarian insufficiency is a change in menstrual cycles. This is very non specific but shorter periods or longer heavier periods can both be seen. Periods may become irregular and further apart. When this has been occurring for at least consecutive 3 months, it’s worth checking an FSH level in the patient.
For example, a female patient under 40 years of age who once had periods lasting 5-7 day but now has 3 day lighter periods for many months must be evaluated further by the doctor for possible incipient premature menopause or primary ovarian insufficiency. Other symptoms include hot flashes, vaginal dryness and dyspareunia (pain during intercourse), dry skin, mouth, eyes, decreased libido, increasing number of urinary tract infections or UTIs, urinary frequency (need to urinate frequently), headaches, poor sleep (insomnia), changing mood, breast tenderness, heart racing, weight gain, problems concentrating, muscle and joint pains and infertility. Finally, hair loss can be a sign of primary ovarian insufficiency (POI) as well, especially an acceleration of an androgenetic alopecia like picture. Hair shedding occurs along with a thinning of hair in the central scalp and sometimes diffusely.
Besides the changes in menstrual cycles noted above, it is important to point out that about 20 % of patients who enter into premature menopause have minimal to no symptoms. So we must take note to ask about menstrual cycles and how they are changing.
Primary Ovarian Insufficiency and Hair Loss
Not all patients with POI or early menopause experience hair loss just like not all women experience hair loss with transition to menopause. However, some patients with POI will experience an acceleration of their underlying androgenetic alopecia (if they have AGA to begin with) and some will experience a shedding phenomenon consistent with a telogen effluvium. In addition, some women will first notice AGA-like hair thinning at the time that a POI is having effects on the rest of the body. I always evaluate for possible POI in women age 30-40 with hair shedding or dramatic changes in their underlying AGA who also report significant shortening of the menstrual cycles or lengthening of the duration between cycles. In my practice, this is most often women ages 30-40 who note that their periods are much shorter (5 or 6 days down to 2 or 3) or much lighter than in previous years. The second group of women who I see are women with longer duration between periods and occasional months with no period at all. These symptoms raise concern for possible POI.
The Importance of Recognizing Early Menopause
Every year, I diagnose primary ovarian insufficiency in a few patients who did not know this was even a concern. There are so many reasons that specialists of all backgrounds need to recognize and ultimately confirm a diagnosis of primary ovarian insufficiency, premature menopause and early menopause. First, many patients I see with a diagnosis of primary ovarian insufficiency have had children and have completed their families. Fertility issues are not key issues for these patients. However, some of my patients with POI would like to have children or would like to have additional children. If we can recognize POI and incipient premature menopause as early as possible, patients who wish to become pregnant can be referred to a gynaecologist for further evaluation and help with plans for future pregnancy. But there are many other reasons that getting the diagnosis is so important. Women with premature menopause or early menopause are at increased risk for cardiovascular diseases, osteoporosis, cognitive issues, type 2 diabetes, Parkinson’s diseases and glaucoma. I encourage patients to see a gynaecologist or endocrinologist and get connected with the latest and most up to date approaches to optimize health.
Evaluation of Early/Premature Menopause/POI.
When I see a patient who I feel might have early or premature menopause or concerns of primary ovarian insufficiency, I make sure they get connected with a gynaecologist as soon as possible. I generally will perform a good history asking about symptoms and family history and then order some basic tests that I know will be helpful to the gynaecologist. These include tests such as estrogen, LH, FSH, testosterone, DHEAS, TSH, Prolactin, B12, glucose and hemoglobin A1c. Sometimes a pregnancy test (beta HCG) is ordered as well in the event that recent cessation of periods is actually due to pregnancy. Menopause is associated with low estrogen (less than 30 pg/mL or 110 pmol/L) and high FSH (above 40 mIU/L). The perimenopausal period is associated with estradiol and FSH numbers somewhere in between.
The AMH Test
When discussing primary ovarian insufficiency and possible early or premature menopause, it’s helpful to know about the AMH test. AMH may become increasingly important in the evaluation of early POI.
AMH is a protein that is made by the ovary. Specifically, it is made by granulosa cells of primary follicle. The AMH is a test that I sometimes will order to better assess the possibility of primary ovarian insufficiency (POI) as I wait for my patient to be referred to a gynaecologist for further evaluation of this possible diagnosis. I generally order AMH if the patient is hoping to have additional children and I want them to see a gynaecologist. This is because the AMH test measures “ovarian reserve” - or the number of follicles remaining in the ovary. Some refer to this test as a measure of “ovarian aging”.
It’s important to keep in mind that there are other ways of measuring “ovarian reserve” besides the AMH but the AMH is among the best ways. These other methods include measuring FSH and estradiol blood tests on the third day of the menstrual cycle and using ultrasound to measure the number of antral follicles that are 2-10 mm in the early follicle phase. All these give some measure of ‘ovarian reserve’. An FSH above 10 IU/L on day 3 is thought to be consistent with “reduced ovarian reserve”.
AMH measurements, however, are currently viewed as one of the best measures of ‘ovarian reserve’ and that is why it’s important to know about the AMH test. Even if FSH and estradiol levels are normal and even if menstrual cycles are regular, a low AMH test result indicates reduced ovarian reserve. That is really important information.
Technically speaking, AMH is actually a measure of the number of AMH producing antral follicles that have been recruited in any given menstrual cycle to produce an egg. But that’s a bit complicated so AMH is most often simply viewed as a measure of the number of eggs remaining in the ovary. As a woman ages, the number of eggs in her ovaries decreases over time - and so does her AMH lab test result. We’ll talk more about how AMH declines in a moment.
AMH declines with age and become undetectable in menopause. AMH levels can also be reduced with a number of other factors like smoking, autoimmune disease, low vitamin D and birth control pills. AMH levels are fairly constant across the menstrual cycle in older women but in younger women they are highest in the follicular phase (first 14 days after menstruation). The key parameter, however, that affects AMH is age. Young patients have higher AMH and more eggs In the ovary and older patients have lower AMH and less eggs in the ovary.
What is a normal AMH Level … and what is considered low?
When a patient asks me “what do you consider to be a normal AMH level” I usually reply by saying “I would just need to know the age of the patient you are referring to first and then I can tell you what the normal AMH level would be for a patent of that age.”
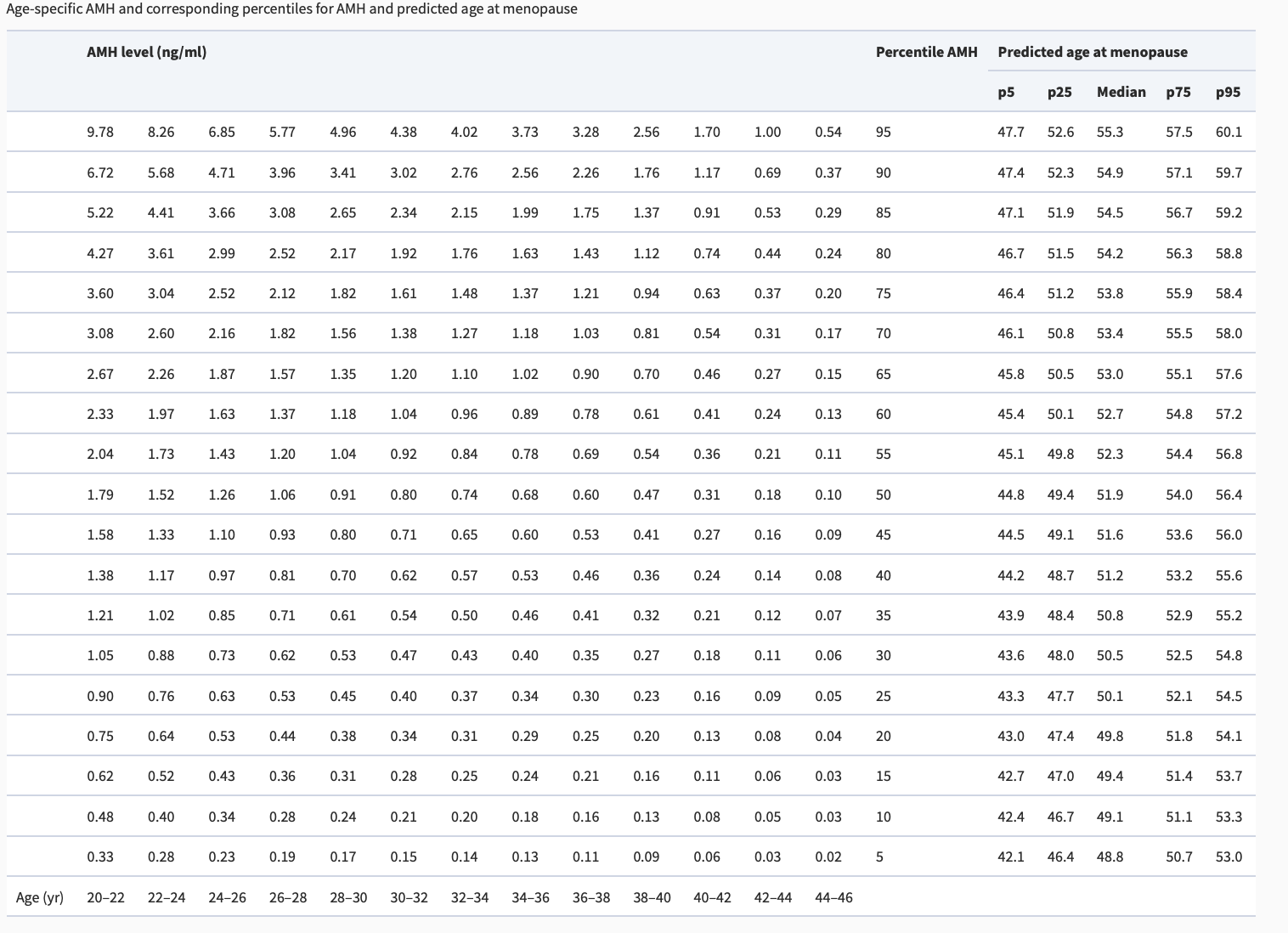
This is because AMH levels vary by age. A normal AMH for a woman in her 20s is above 5 ng/mL (38.5 pmol/L). This would not be considered a normal AMH level for a woman in her late 30s. A woman in her late 30s would typically have a an AMH around 1.5 ng/ml (10.7 pmol/L)
As you can see in the above chart, it’s not really appropriate to speak in terms of normal AMH and abnormal AMH levels without referencing the age of the patient. A normal AMH level for a woman in her 40s would be considered abnormal for a woman in her 20s.
Generally speaking however, a cut off of 0.7 ng/mL (5 pmol/L) is often set for “low AMH” vs above 0.7 ng/mL as normal AMH. (5 to 60 pmol/L). Other specialists have slightly different ranges but the basic message is still roughly the same. For example, an AMH in the range of 0.5 to 1.5 ng/mL is often said to be a “normal-low” AMH range and an AMH less than 0.5 ng/mL is said to be low AMH. An AMH of 1.5 to 3 ng/mL is said to be a normal range. Other researchers use a cut off of 1 ng/ml (7.1 pmol/L) as indicative of “reduced ovarian reserve.”
AMH is often used the reproductive endocrinology field as a measure of the chance of having successful in vitro fertilization (IVF). Therefore, a low AMH test result has a specific meaning in the IVF clinic. Some gynaecologists who specialize in helping women achieve pregnancy through IVF often look carefully at the AMH values. Women with AMH levels less than 0.5 ng/mL and especially less than 0.2 ng/mL have a more difficulty time achieving sufficient eggs through IVF. The thing about AMH is that conclusions based on AMH are never an absolute - and many women with low AMH still have successful pregnancy and IVF cycles. But these numbers provide a guide.
Why do I order AMH?
AMH has been helpful in my practice in situations where the patient still has somewhat regular periods but I suspect the patient might have primary ovarian insufficiency (POI) and FSH fluctuates between high and high normal. These are often situations whereby I am suspecting POI and need some further evidence to get my patient the help I feel they need. Often the patient in this situation is in her early to mid 30s who comes to see my with concerns about hair loss but who is noted to have lighter periods and some of the above symptoms of premature menopause.
The AMH test is complex because it has to be ordered in the right context or else if just gives false information and unnecessary worry. There are a number of additional points that are worth mentioning about AMH and I’ll highlight them here.
a) Women with low AMH levels can still become pregnant so low AMH does not mean the patient will not be able to become pregnant.
Studies from 2017 from the journal JAMA taught the world that ordering the AMH test in women 30-44 without fertility issues just because we think we should order it has limited use. It does not predict how easy it will be for the patient to become pregnant any time soon. Women with low AMH (defined in this particular study as an AMH less than 0.7 ng/mL) and normal ranged AMH (defined here as AMH above 0.7 ng/mL) seemed to have similar chances of becoming pregnant. The same was true for women with FSH results less than 10 and above 10.
b) In the right context, a lower AMH level indicates lower “ovarian reserve” and gives a very very appropriate estimate of when menopause is likely to occur.
It’s still somewhat controversial exactly how AMH can be used to predict the timing of menopause. Broer and colleagues noted that the level of AMH switches to very low, or even below detectable limits approximately 5 years before menopause.
In some studies, AMH levels seem to be highly predictive for the timing of menopause. In the right clinical context however, a low AMH level in young women may be suggestive of a greater risk for premature menopause. We’ll take a look at that in a moment.
If the day 3 FSH test result comes back high (in the 30s) and day 3 estrogen is low , I don’t generally order AMH because the clinical results already suggests a high likelihood of transition to an earlier menopause. I often refer to gynaecology at that point. I may order AMH in a patient with significant change in her cycles who has day 3 FSH in the 15-30 range in whom I am concerned about early POI. Of course, if I have concerns of any kind I usually refer my patients to trusted gynaecologists.
The AMH is NOT a perfect test by any means. However, the more and more that AMH falls below normal levels, the greater and greater risk that the patient has for entering into menopause in the years ahead especially if she has clinical symptoms suggestive of possible POI. It still could be 5, 10 or 15 years before the patient with a low AMH test result will enter menopause but the age of menopause gets moved up the lower and lower that the AMH falls. For a female patient who comes to see me for hair loss, but who indicates during the appointment that she wants to have children or have additional children, this is an important test especially if day 3 FSH and estradiol are not completely inn the menopausal ranges and periods are somewhat irregular but a number of clinical symptoms that she has raise concerns about POI.
A 2012 study by Freeman and colleagues suggested that women with lower AMH enter menopause sooner than women with higher AMH. In that study women age 35-39 with AMH greater than 1.5 ng/mL experienced menopause about 13 years later. However, similar aged women with AMH less 0.2 ng/ml experienced menopause in about 9.94 years - i.e. about 3 years earlier than the first group. .
A 2011 study by Broer and colleagues is a study that I refer too often. The authors studied 257 women 21-36 and two time points 11 years apart. The conclusion of this study was that AMH together with the age of the patient are predictive of menopause. Table 3 from Broer’s paper is very helpful. It allows one to predict the approximate age range that patient’s will enter menopause based on their current age and AMH. The age ranges vary widely which just shows us that predicting menopause is still quite challenging and that use of AMH and age alone to help us predict the age of menopause only gets us so far.
Several studies have examined the relationship between AMH levels and the risk of premature menopause. The 2018 study by Berton and colleagues was one of the first prospective studies to evaluate AMH levels in healthy women ages 35-44. The study showed an association between low AMH levels and the risk of early menopause. For every 0.1 ng/mL drop in AMH levels below 2 ng/mL (ie 0.7 pmol/L) there was a 14 % increased risk of early menopause.
SUMMARY and CONCLUSION
I’ve learned a lot about ovarian insufficiency over the years. Every year, I diagnose POI in several patients who come and see me for a consultation about hair loss. I keep a low threshold for asking myself - could this be POI? I ask about a family history of early menopause in every female patient I see. Family history is a really important risk factor for POI and early menopause. I ask about menstrual cycles in every female patient that I see. Significant changes in cycles for more than 3 consecutive months are concerning. I ask about hot flashes, vaginal dryness, dry skin, mouth, eyes, decreased libido, increasing number of urinary tract infections, urinary frequency (need to urinate frequently), headaches, poor sleep, changing mood, breast tenderness, heart racing, weight changes, problems concentrating, and muscle and joint pains in every patient that I see.
When I have even the slightest concern about possible POI, I order a day 3 FSH and estradiol. If FSH comes back high, I refer to my gynaecology colleagues. If I’m concerned something just does not “seem right”, I refer patients to my gynaecology colleagues. Sometimes I order an AMH for my patients but my preference is to have these tests ordered and interpreted by my gynaecology colleagues - if possible. They can review the patent’s full story and symptoms and order additional blood tests and perform a transvaginal ultrasound to look for antral follicles. They can order AMH from labs they trust and follow these numbers with patients over time if there is any concern. If POI is confirmed and pregnancy is the goal of the patent, the gynaecologist can assist the patient with a number of reproductive technologies. If POI is confirmed and pregnancy is not the goal, the endocrinologist or gynaecologist can assist with strategies to reduce the risk of heart disease, osteoporosis and cognitive changes. Most often this is with hormone replacement therapy which is used until age 50-51.
REFERENCE
Bertone-Johnson. Anti-Müllerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reprod. 2018 Jun; 33(6): 1175–1182.
Broer SL et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update.
Freeman EW et al. Anti-Müllerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680.
Shebi O et al. Age-related distribution of basal serum AMH level in women of reproductive age and a presumably healthy cohort. Fertil Steril. 2011 Feb;95(2):832-4.
Steiner AZ et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age.JAMA. 2017;318(14):1367-1376. doi:10.1001/jama.2017.14588
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.