What is Gynecomastia?
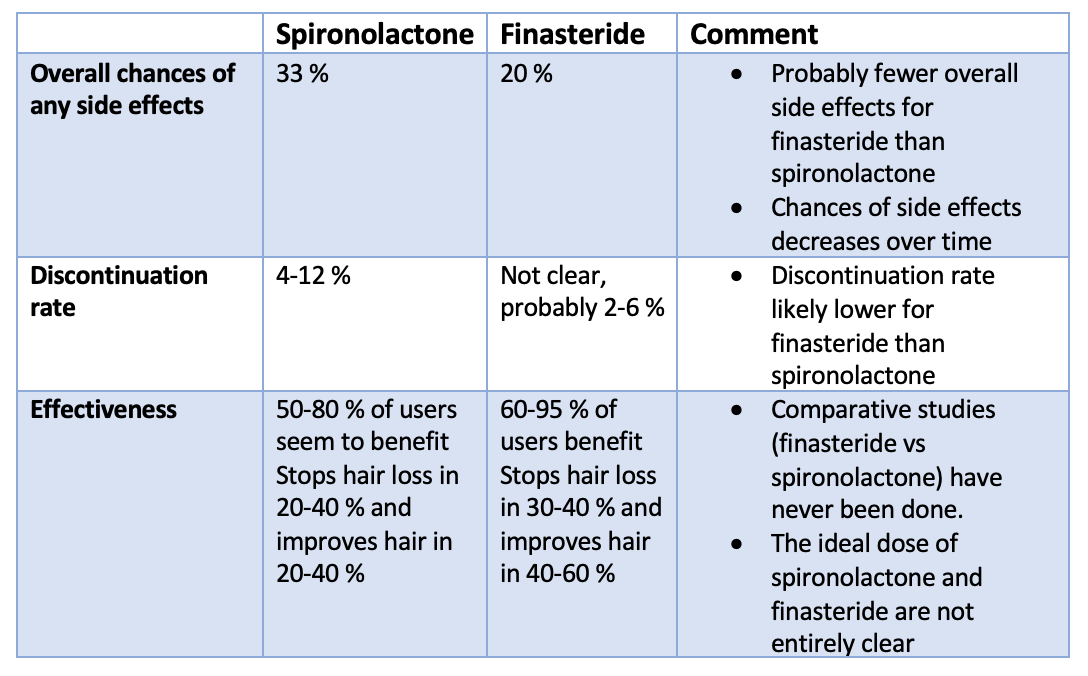
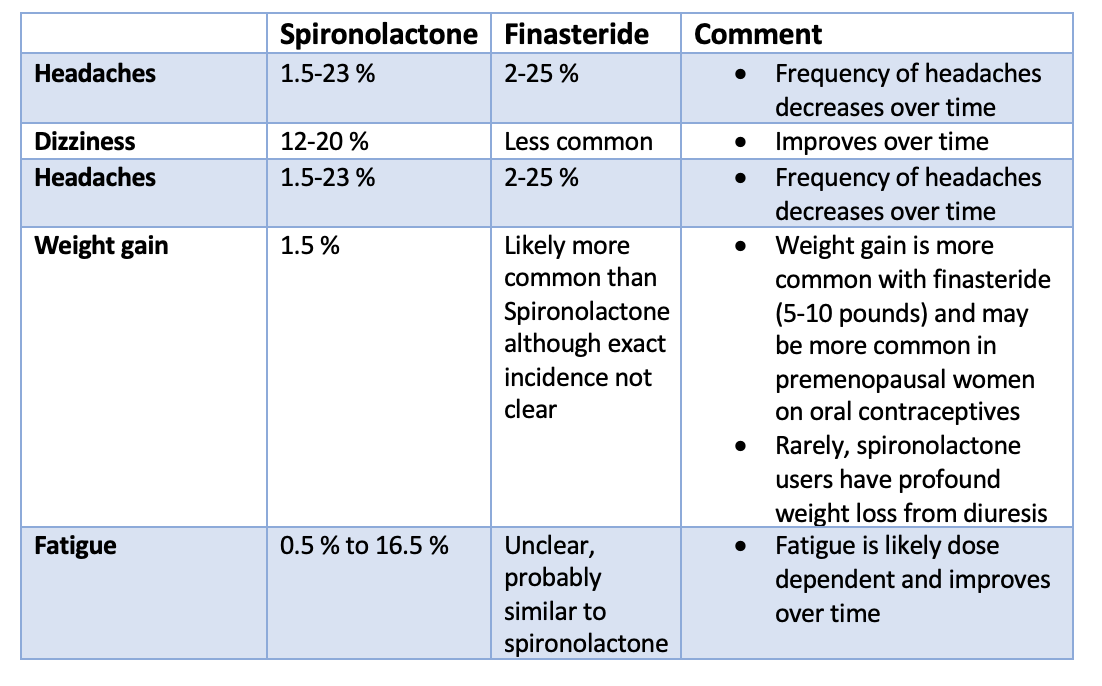
Gynecomastia refers to the enlargement of breast tissue in men. It's extremely common and throughout life, a majority of men will be affected. For some, it will occur during infancy or puberty and resolve. For others it will start in puberty and persist and for others it will occur in older ages.
Gynecomastia by Age
Gyneomastia is common in newborns. Due to estrogens from the mother, 60-90% of male babies have enlarged swollen breast tissue. It resolves in a matter of weeks for most. For young male adolescents, gynecomastia is embarrassing but common. It can start at age 10-12 but typically it starts around 13 or 14 years of age. About 50 % of male adolescents may be affected. The breast swelling goes away on its own in 6 months to 2-3 years. In 20 % of affected teens, the swelling persists into adulthood. In men, gynecomastia is present in men 20-40, but particularly peaks in incidence after age 50. In the 50 plus age group, about 25-30 % of men are affected. The number may rises to well over 50 % in 60 + age groups.
What causes gynecomastia?
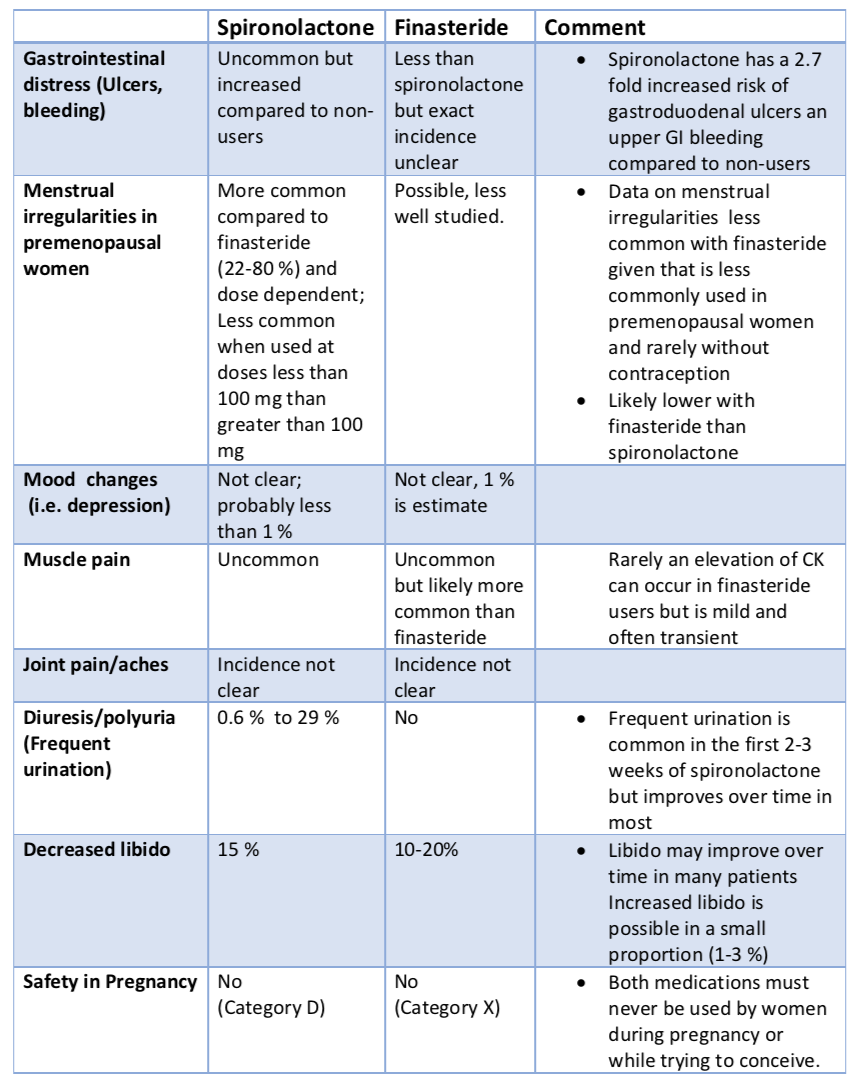
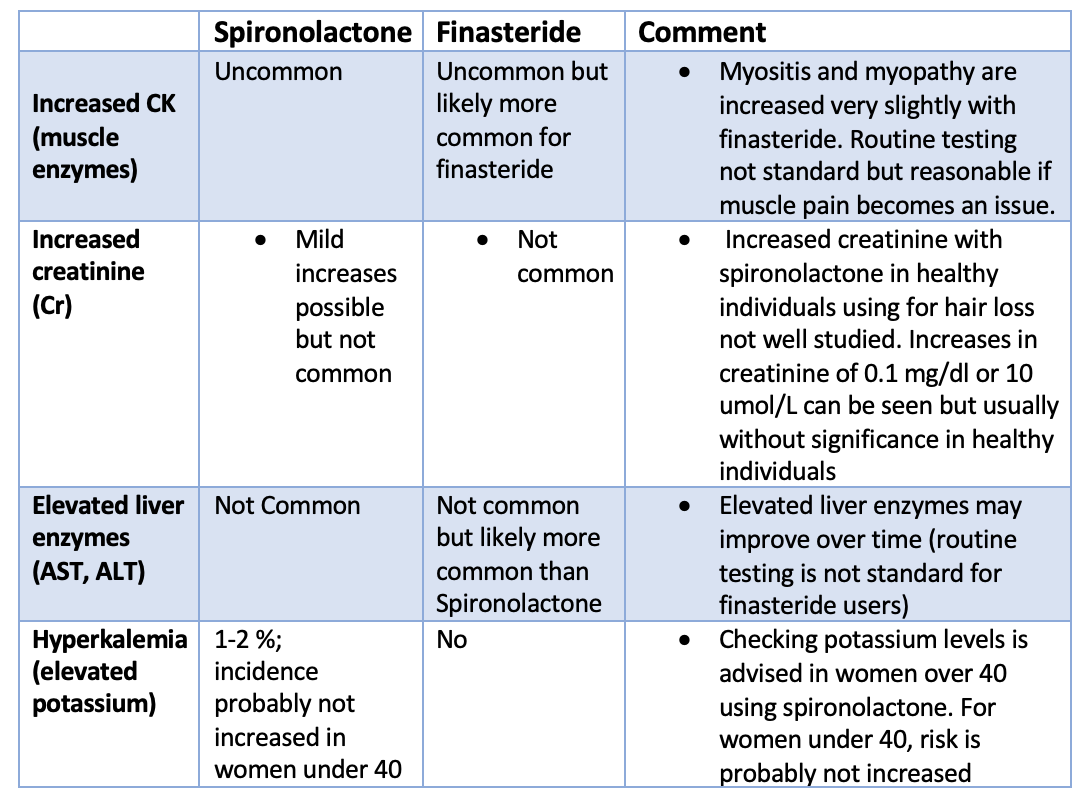
Gynecomastia is caused by a variety of factors including genetics, drugs and physiological causes. Regardless of the precise cause, it is though that some hormonal imbalance (especially favouring estrogen and increasing the estrogen to androgen ratio) triggers breast tissue enlargement. The actual level of hormones in the blood stream is typically normal and it's the ratio of these drugs at the actual level of the breast tissue itself that is important. Drugs cause about 10-25 % of cases. Pubertal gynecomastia that persists into adulthood is responsible for another 25 % of cases. The causes is generally unknown in about 25 % of cases as well. But the list of potential causes is long and a full review is needed for anyone with concerns about gynecomastia. A variety of genetic syndromes (i.e. Klinefelter syndrome), metabolic problems, cancers all reduce the bodies production of testosterone and increase the estrogen to androgen ratio.
Drugs causing Gynecomastia
For hair specialists, it's well know that finasteride is among the causes of gyneocmastia. About 4 to 10 out of every 1000 men using finasteride will develop gynecomastia on account of the drug. But a variety of other medications and products can cause gynecomastia including:
1. Anti-androgens. (Finasteride Dutasteride). This anti-androgens is commonly used for treating male pattern balding.
2. Anti-androgens. (Spironolactone). This anti-androgens is typically used for women with hair loss but not typically for men.
3. Ketoconazole (anti-fungal). Generally speaking the risks is greatest with oral ketocoanzole rather than topical products. Oral ketoconazole is rarely used nowadays in North American due to safer anti-fungal agents.
4. Heart burn medications (H2-receptor blockers). Drugs used to treat ulcers and heart burn including Cimetidine has the greatest evidence as a drug of this class being implicated. The others less so.
5. Tea tree oil and lavender oils. These essential oils have been reported to increase the risk of gynecomastia in children. Tee tree and lavender are found in soaps, shampoos and lotions.
6. Other drugs. A variety of other drugs may also be implicated. A 2012 review classified drugs into two main groups including those 'definitely associated with gynecomastia" and those "probably associated with gynecomastia." The first group (drugs definitely associated with the onset of gynecomastia) inlcuded spironolactone, cimetidine, ketoconazole, hGH, estrogens, hCG, anti-androgens, GnRH analogs and 5-α reductase inhibitors. The second group of drugs "probably associated with gynecomastia" include risperidone, verapamil, nifedipine, omeprazole, alkylating agents, HIV medications (efavirenz), anabolic steroids, alcohol and opioids.
Finasteride Induced Gynecomastia
Finasteride is FDA approved for treating male balding at a dose of 1 mg daily. It is among the medications known to cause gynecomastia. The risk is likely about 4 to 10 out of every 1,000 users. Finasteride induced gynecomastia is often one-sided but can be both sides. This is especially true at lower doses like 1 mg compared to 5 mg. Finasteride induced gynecomastia can start as early as a 1-2 weeks after staring the drug but is typically a few months delay. It can also be 1-2 years before the phenomenon is appreciated. Pain is not uncommon in men with finasteride induced gynecomastia - and this pain/tenderness can occur prior to any actual enlargement. The 1 mg dose is less likely than the 5 mg dose to cause breast enlargement in men. Finasteride induced gynecomastia can reverse completely in many individuals provided the drug is completely stopped in the early stages when the breast enlargement is noted. If the drug is not stopped, it can enter a irreversible stage (where only surgical treatment will provide treatment). Finasteride induced gynecomastia is more likely in obese indivdiuals and with advanced age. Most of the time blood tests and various hormonal tests are normal in men with finasteride - induced gynecomastia.
Treatment: How is gynecomastia treated?
The treatment of gynecomastia depends on the precise causes. For most adolescents, a conservative (watch and wait) approach is usually taken as the condition usually resolves in most cases. Only 20 % of adolescent cases of gynecomastia persist into adulthood. Medical treatments are usually not effective. However, in some hormonal abnormalities, medications such as aromatase inhibitors can be used. Usually surgery is needed to remove the excess tissue if that is what is so desired by the patient.
REFERENCES
Rodriguez et al. Risk of gynaecomastia associated with cimetidine, omeprazole, and other antiulcer drugs. BMJ. 1994 Feb 19; 308(6927): 503–506.
Bera F, et al. [Impotence and gynecomastia secondary to hyperprolactinemia induced by ranitidine].Therapie. 1994 Jul-Aug.
Deepinder F, et al. Drug-induced gynecomastia: an evidence-based review. Review article. Expert Opin Drug Saf. 2012.
Fentiman IS, et al. Managing Male Mammary Maladies. Eur J Breast Health. 2018.
Soliman AT, et al. Management of Adolescent Gynecomastia: An Update.Acta Biomed. 2017