Hair loss from CGRP Monoclonal Antibodies for Migraine Treatment
New study identifies hair loss in 2 % of patients using CGRP monocloncal antibodies
introduction: CGRP and Its Role in Migraine
Migraine is a highly prevalent and disabling disorder, with an estimated 1 in 5 women and 1 in 15 men in the United States affected by migraines.
In the current model for migraine pathogenesis, neurons on dural blood vessels trigger the release of plasma proteins and vasoactive substances, including calcitonin gene-related peptide (CGRP), substance P, and neurokinin A. The release of vasoactive factors triggers vasodilation and dural plasma extravasation, resulting in neurogenic inflammation and thereby producing pain.
Calcitonin gene-related peptide (CGRP) is now thought to play a key role in migraines. CGRP levels are elevated in serum between attacks of episodic and chronic migraine and this increase is then reversed upon treatment with standard first-line treatments like triptans. It is well known that injection of the CGRP peptide triggers moderate to severe headaches similar to typical migraine.
CGRP Targeting Drugs
Calcitonin gene-related peptide (CGRP) inhibitors block the effect of CGRP. Calcitonin gene-related peptide-targeted drugs have proven safe and effective for migraine prevention in large randomized-controlled, double-blind trials with an average duration of six months.
There are two types of CGRP inhibitors – monoclonal antibodies and CGRP receptor antagonists (gepants).
The monoclonal antibodies include:
• Aimovig (erenumab): Approved May 17, 2018
• Ajovy (fremanezumab): Approved Sept 14, 2018
• Emgality (galcanezumab): Approved Sept 27, 2018
• Vyepti (eptinezumab): Approved Feb 21, 2020.
The “gepants” include:
• Ubrelvy (ubrogepant): Approved Dec 23, 2019
• Nurtec ODT (rimegepant sulfate): Approved Feb 27, 2020.
• Qulipta (atogepant): Approved Sept 28, 2021
• Zavegepant (Zavzpret): Approved March 10, 2023)
Evers et al, 2023
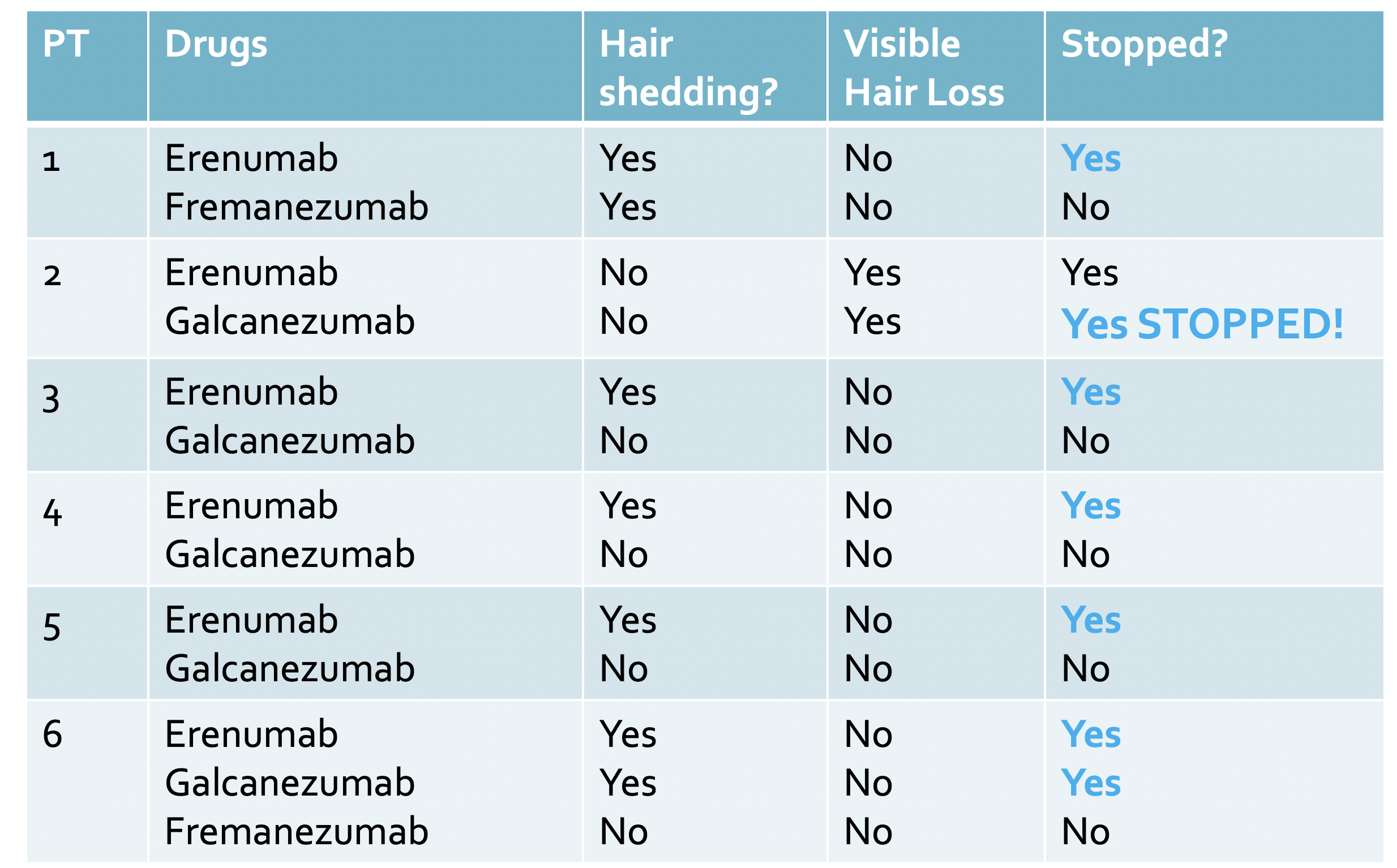
Authors from Germany described 6 patients out of 279 patients treated monoclonal CGRP antibodies over a 3 year period. That equates to 2.2 %
All patients were female patients with chronic migraine. Ages of patients ranged from 40-62 years. Hair loss due to iron deficiency and thyroid problems was ruled out. All patients started with erenumab. 5 of the patients had excessive shedding and 1 patient developed a visible hair loss (rather than a shedding).
The hair loss started between 1 and 4 weeks after starting treatment with the monoclonal CGRP antibody. All patients switched to another monoclonal CGRP antibody. In four of these patients, the effluvium stopped completely! In the other two patients’ alopecia or effluvium also occurred with the second monoclonal CGRP antibody. Of these two patients, one stopped treatment with monoclonal CGRP antibodies and one switched to a third monoclonal CGRP antibody after which effluvium finally stopped.
Discussion
This is a helpful paper which puts hair loss at around 2-3 % of patients treated with erenumab. This drug remains one of the most common of the CGRP monoclonal antibodies to be implicated in hair loss. Part of this simply may be due to the fact that it is often the first drug to be prescribed. This drug was FDA approved in 2018 and is marketed in many counties as Aimovig. It was the first CGRP monoclonal antibody to be approved.
This study teaches us that it’s worth making a switch to another anti-CGRP type treatment if shedding is troublesome or hair loss has occurred. In many patients, this switch is expected to resolve the hair loss issue. If not, another switch is worth it!
REFERENCE
Donovan J. Hair Loss from the CRGP Monoclonal Antibodies
Evers S and Wald S. Effluvium and alopecia associated with monoclonal calcitonin gene-related peptide antibody use. Headache. 2023 Jan;63(1):165-167.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.