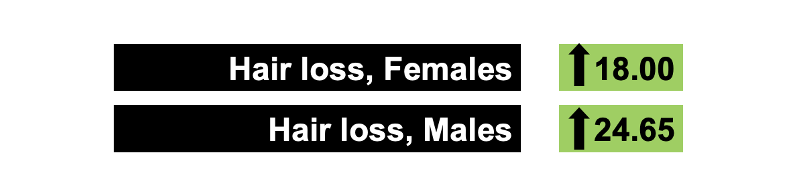New Study Points to Distinct Hair Loss Concerns with Multiple Sclerosis Drug Teriflunomide
Multiple sclerosis is an immune mediated disease of the nervous system. The disease leads to damage to myelin, which is a protective layer around nerves. The result of such damage is a variety of neurological symptoms such as numbness, tingling, mood change, pain, fatigue, memory issues, blindness and weakness.
There are now about 20 or so disease modifying therapies (DMTs) available to treat MS. DMTs target some component of the inflammatory process of MS and help to either reduce the frequency and severity of relapses, reduce the number of new lesions in the brain and spinal cord as seen on MRI, and/or slow down the accumulation of disability.
There have been reports of hair thinning in patients with multiple sclerosis (MS) treated with various disease-modifying therapies (DMTs). In a prior article, I reviewed the potential for the drug teriflunomide to cause hair loss.
Porwal MH et al. 2022
Authors set out to analyze total reports, source of reporting, age distribution, and sex distribution of alopecia associated with disease modifying therapies.
To do so, authors used an FDA Adverse Event Reporting System (FAERS) public dashboard and OpenFDA database to analyze reports of hair loss between January 1, 2009, and June 30, 2020, attributed to usage in MS of FDA approved DMTs. Reports in the FDA Adverse Event Reporting System (FAERS) and OpenFDA database are voluntarily submitted to the FDA by patients and healthcare professionals and are accessible to the general public.
Key Results
There were 8759 alopecia reports in the database among 44 114 adverse events in skin and subcutaneous tissue disorders (19.9%). Reports were mostly made by patients (78.3%) rather than doctors and highest in fifth and sixth decades of life.
The breakdown of reports of hair loss were as follows:
3701 (42.3%) with teriflunomide (the active metabolite of the immunosuppressant leflunomide).
1675 (19.1%) with dimethyl fumarate (an immunomodulatory medication to suppress inflammation)
985 (11.2%) with natalizumab (the humanized monoclonal antibody against the cell adhesion molecule α4-integrin)
926 (10.6%) with fingolimod (a sphingosine-1-phosphate receptor modulator, which sequesters lymphocytes in lymph nodes)
659 (7.5%) with interferon beta-1a (an immunomodulatory cytokine)
257 (2.9%) with glatiramer acetate (a synthetic protein that simulates myelin basic protein, a component of the myelin that insulates nerve fibers in the brain and spinal cord)
243 (2.8%) with ocrelizumab (a humanized anti-CD20 monoclonal antibody)
124 (1.4%) with interferon beta-1b (an immunomodulatory cytokine)
117 (1.3%) with alemtuzumab ( a drug depleting CD52-bearing B and T cells)
36 (.4%) with siponimod (selective sphingosine-1-phosphate receptor modulator)
24 (.3%) with cladribine, (a purine analogue that selectively targets and suppresses lymphocytes implicated in the underlying pathogenesis of MS as well as B-cell leukaemia
12 (.1%) with rituximab. (an anti-CD20 monoclonal antibody)
Teriflunomide Stands out as Having 20 fold Increased Risk for Hair Loss
OpenFDA analyses showed increased odds ratio (ROR 95% confidence interval) of alopecia in females with teriflunomide (18.00, 17.12-18.93), alemtuzumab (1.43, 1.16-1.76), dimethyl fumarate (1.26, 1.18-1.34), and ocrelizumab (1.28, 1.11-1.49). Increased risk in males was associated with teriflunomide (ROR 24.65, 20.72-29.31).
Discussion and Comments
This study highlights the disproportionate reporting of hair loss with teriflunomide in both males and females. There may be risk with other drugs such as alemtuzumab, dimethyl fumarate, and ocrelizumab - especially in females. However, the risk is particularly different with teriflunomide.
It’s interesting that most reports of hair loss are submitted by patients rather than health care professions. One of the problems of many of these types of databases is the way that data gets entered. In voluntary reporting systems, patients may often drive data collection of side effects such as hair loss.
Reference
Porwal MH et al. Alopecia in Multiple Sclerosis Patients Treated with Disease Modifying Therapies. J Cent Nerv Syst Dis. 2022 Jun 23;14:11795735221109674.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.