DPCP for Children and Adolescents
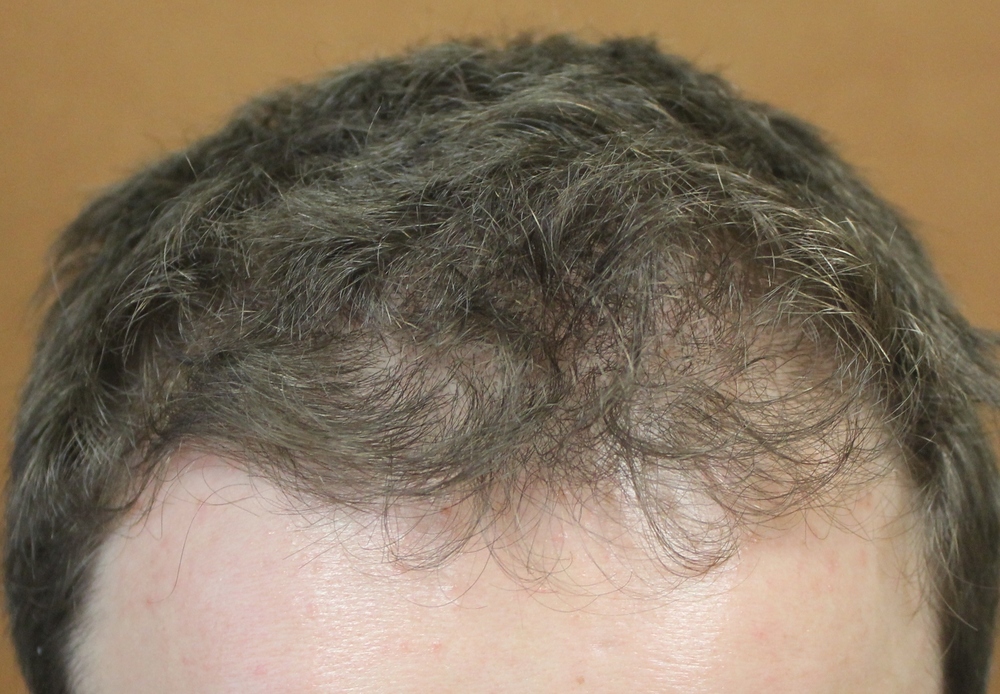
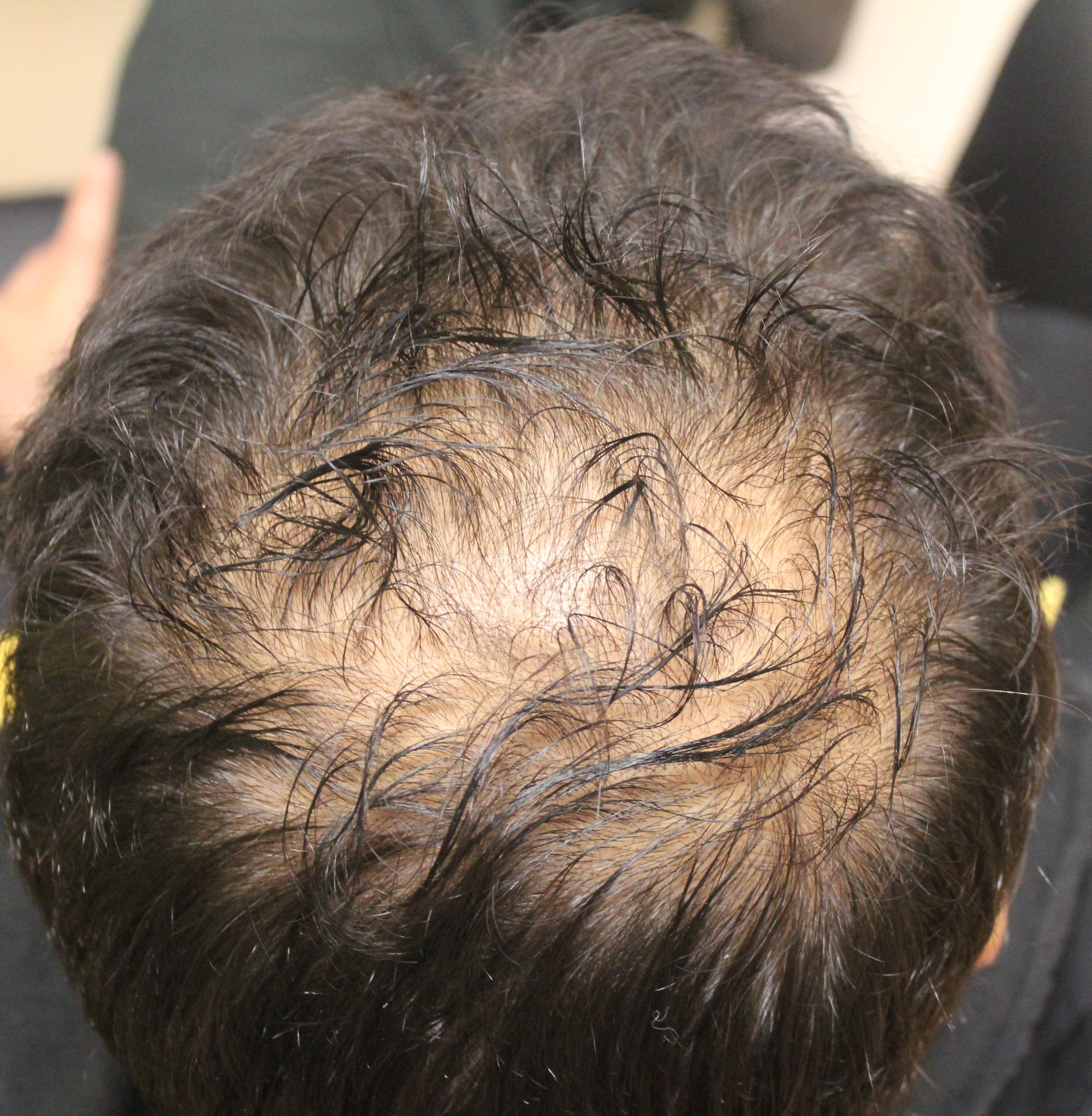
Diphencyprone or “DPCP” is frequently prescribed for individuals with alopecia areata who develop more extensive amounts of hair loss or for individuals who aren’t improving with steroid injection treatments. As shown in the photo to the right, DPCP is a liquid and is applied to the scalp weekly, usually in a dermatology clinic setting. It causes a mild allergic reaction in the scalp skin, which in turn promotes hair regrowth in some individuals. In adults, DPCP treatment promotes hair regrowth in approximately 30-50 % of individuals.
What about DPCP in Children & Adolescents?
We decided to examine this question. Prior to our study, the use of DPCP in children had not been thoroughly explored is whether DPCP is effective for children with alopecia areata. In fact, the use of DPCP in children has been the focus of only a 3-4 of research studies - and these studies were quite small. One previous research study of 26 children indicated that DPCP helped with hair regrowth in 35% of patients. A second study of 12 patients indicated hair re-growth in 67% of patients.
We recently published our research findings in the journal Archives of Dermatology. We looked back through the medical charts of 108 children who received DPCP at Sunnybrook Hospital in the past 10 years. Children ranged in age from 4 months to 18 years. Most children had tried other treatments, such as steroids or minoxidil, prior to starting DPCP. However, none of those treatments were helpful and so DPCP was started.
Does DPCP have side effects in Children and Adolescents?
Overall, treatment with was safe, but minor side effects did occur in about one-half of patients. These included swelling, hives, small blisters and skin breadkdown and swollen lymph nodes. About 13 % of patients stopped treatment after 2 months owing to a variety of factors, such as these side effecsts, difficulties commuting to the treatment center, and/or the disruption caused by weekly absences from school.
Was DPCP Beneficial ?
Overall, our research data showed that about one-third of children benefitted from DPCP treatment. 25 % of children had a partial improvement and 10 % had full regrowth.
Conclusion
Our study is one of the largest research studies looking at whether DPCP is beneficial for children and adolescents with alopecia areata. It is a valuable study because it provides us helpful information that we can share with parents who bring their child to the DPCP clinic. Overall, DPCP will help about 1 out of every 3 children who go through treatment. However, only 1 out of every 10 children will experience full regrowth with treatment. Right now, it’s not possible to predict which children will benefit from DPCP and who will not. Certainly, more research is needed to understand how to make DPCP even more effective for children.
References of Interest
1. Salsberg, J and Donovan, J. The Safety and Efficacy of Diphencyprone for the Treatment of Alopecia Areata in Children. Archives of Dermatology 2012; 148: 1084-5.
2. Schuttelaar ML, Hamstra JJ, Plinck EP, et al. Alopecia areata in children: treatment with diphencyprone. Br J Dermatol. 1996;135(4):581-585.
3. Hull SM, Pepall L, Cunliffe WJ. Alopecia areata in children: response to treatment with diphencyprone. Br J Dermatol. 1991;125(2):164-168.
4. Mukherjee N, Burkhart CN, Morrell DS. Treatment of alopecia areata in children. Pediatr Ann. 2009;38(7):388-395.