JAK Inhibitors for Alopecia Areata: A Closer Look
Which JAK Inhibitors Hold Promise for Treating Alopecia Areata
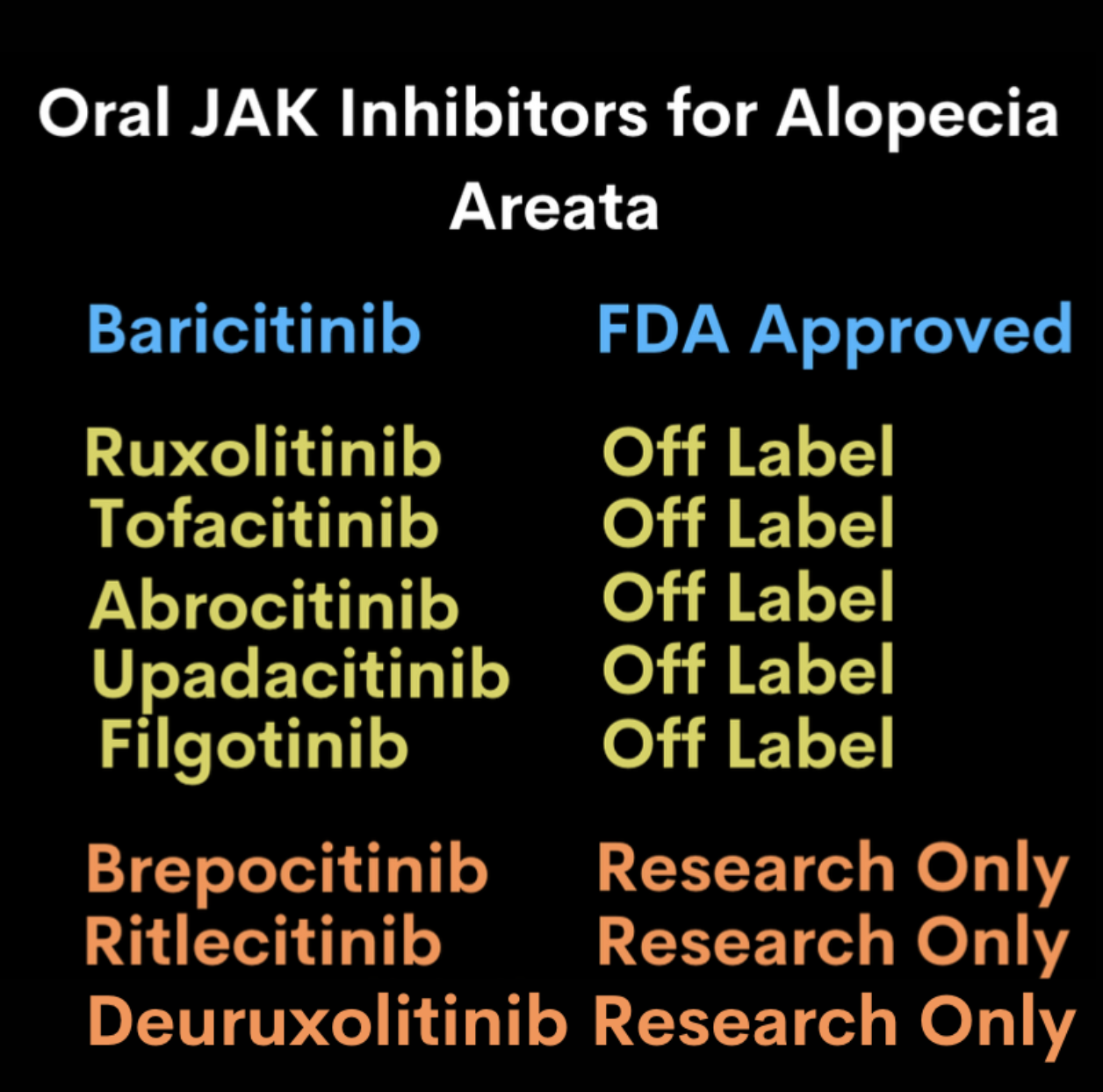
JAK inhibitors are rising to the top of the list as good options for treating severe alopecia areata. To date, six JAK inhibitors have been used for patients with advanced alopecia areata in the real world clinical setting. These include tofacitinib, ruxolitinib, baricitinib, abrocitinib, upadacitinb and more recently filgotinib in Europe.
On June 13, 2022 baricitinib became the first FDA approved JAK inhibitor for treatment of severe alopecia areata in adults.
A number of JAK inhibitors including the three shown in the bottom row have been studied in clinical trials in the last few years. They are not available for routine clinical use at present because no health agency has yet reviewed them to give such approval. These include ritlecitinib, deuruxolitinib and brepocitinib. Ritlecitinib and deuruxocitinib are the two most likely JAKs to be considered for approval in the future.
Several newer JAK inhibitors are being studied including Jaktinib in China and soon Deucravacitinib in centers throughout the world.
JAK Inhibitor Side Effects
JAK inhibitors tend to be fairly well tolerated in patients with alopecia areata. Infections, changes in blood counts, changes in cholesterol, increased CK levels and acne are some of the more common issues seen on a day to day basis in the clinic. Blood clots, cancer and heart disease are issues that a discussed in rheumatology following the completion of the ORAL Surveillance Trial. Blood clots have not been something seen in alopecia patients using JAK inhibitors in trials. But clinical studies, of course, are in the hundreds not thousands and we need big studies. Studies of JAK inhibitors in alopecia areata are not long enough duration to really make any inferences about cancer risk and cardiovascular disease. Certainly cardiovascular disease risk seems extremely low but longer term studies will be needed to prove whether any risk exists at all in patients with alopecia areata. Baricitinib does not seem to cause cancer in animal models. The ORAL Surveillance Trial suggested an increase risk of cancer in rheumatoid arthritis patients using JAK inhibitors. We have no clear indication if this is relevant to our alopecia areata patients or not.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.