The Baricitinib 52 Week Data: Not Much of a Jump from the 36 week Data
New Data Shows Only Mild Increase in Responses with Baricitinib at Week 52 Compared to Week 36
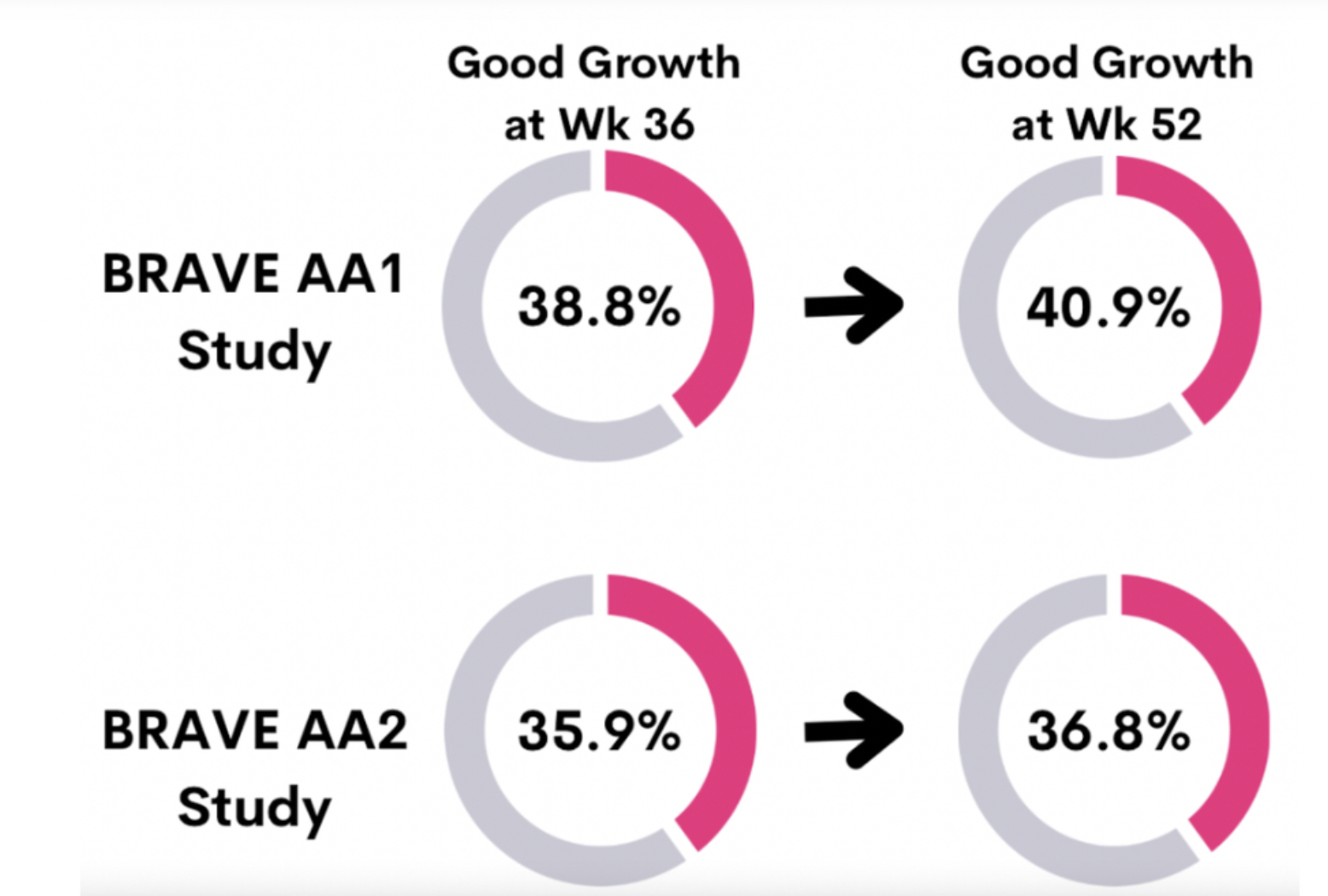
Baricitinib was FDA approved on June 13, 2022 for the treatment of severe alopecia areata (meaning more than 50 % loss). It is a JAK1/JAK2 inhibitor. It was the data from the 36 week “BRAVE AA1” and “BRAVE AA2” trial that led to the FDA approval of baricitinib. The “BRAVE-AA” trials were studies that assessed the benefits of 2 mg, 4 mg baricitinib or placebo in patients with severe alopecia areata. These data showed that 38.8% of patients in BRAVE AA1 and 35.9% of patients in BRAVE AA-2 met the end point of a SALT Score less than 20 (80 % hair coverage or better). Remember, these patients all started out with severe hair loss.
All patients who completed the 36-week placebo-controlled period entered an extension phase, whereby they continued to use the medication.
In a new study, authors report data in patients who used baricitinib 2 mg or 4 mg for 16 more weeks, bringing the total to 52 weeks of continuous treatment . This included 465 patients in BRAVE-AA1 and 390 patients BRAVE-AA2. Overall, 4 more months of treatment increased the proportion of patients achieving the SALT 20 score very slightly - but not as much of a jump as one might have thought.
The data shows here how this 52 week data compares to the 36 week data for 4 mg dosing.
Baricitinib was fairly well tolerated. There were no blood clots in trial participants. There are three cancers so far in the 36 and 52 week data, including ductal carcinoma in situ, squamous cell carcinoma and B cell lymphoma but the authors state it’s not clear if these are coincidental or not.
All in all, about 40 % of patients with severe forms of alopecia areata get back at least 80 % of the hair. Results don’t change all that dramatically from week 36 to 52 indicating that the most dramatic changes take place in the first 6-9 months.
REFERENCE
Kwon O et al. Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata over 52 Weeks of Continuous Therapy in Two Phase III Trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023 Mar 1;1-9.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.