4 more Months of Baricitinib Data: No Blood Clots, 2 New Cancers, Slightly More Patients Meeting Primary End Points
Baricitinib Extension Trial Has Import Lessons For Safety and Efficacy
Baricitinib was FDA approved in June 2022 for the treatment of severe alopecia areata. It is a JAK1/JAK2 inhibitor. It was the data from the 36 week BRAVE AA1 and BRAVE AA2 trial that led to the FDA approval of baricitinib on June 13, 2022. This was a study that assessed the benefits of 2 mg, 4 mg baricitinib or placebo in patients with severe alopecia areata (defined as more than 50 % scalp hair loss). These data showed that 38.8% of patients in BRAVE AA1 and 35.9% of patients in BRAVE AA-2 met the end point of a SALT Score less than 20.
All patients who completed the 36-week placebo-controlled period entered an extension phase, whereby they continued to use the medication.
Kwon O et al. 2023
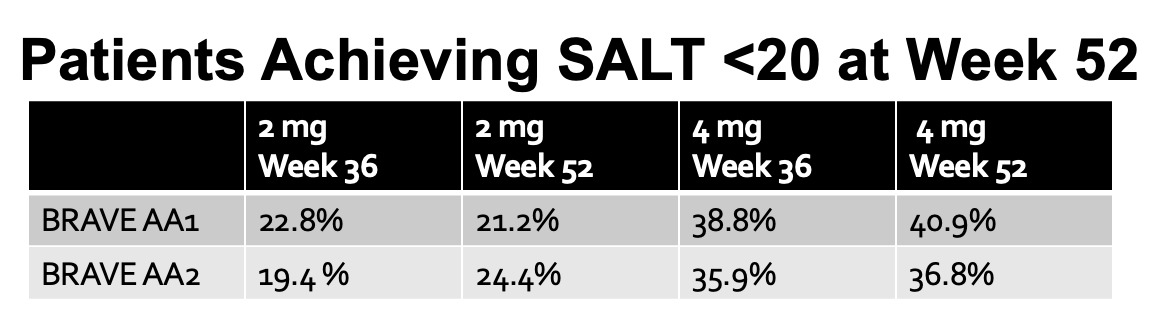
In a new study, authors report data in patients who used baricitinib 2 mg or 4 mg for 16 more weeks, bringing the total to 52 weeks of continuous treatment . This included 465 patients in BRAVE-AA1 and 390 patients BRAVE-AA2. Overall, 4 more months of treatment increased the proportion of patients achieving the SALT 20 score very very slightly. Of patients treated with baricitinib 4 mg and 2 mg, respectively, 40.9% and 21.2% in BRAVE-AA1 and 36.8% and 24.4% in BRAVE-AA2 achieved a SALT score ≤ 20 at Week 52.
The following table shows how this 52 week data compares to the 36 week data.
Side Effects.
Side effects included the side effects reported in the 36 week trial including upper respiratory tract infection, headache, nasopharyngitis, acne, urinary tract infection, increased blood creatinine phosphokinase (CPK), and COVID-19 infection and herpes zoster.
There were 2 more cancers in the extension study bringing the total to 3 cancers so far in the BRAVE trials. There was one B cell lymphoma in BRAVE AA2 36 week trial. In the 52 weeks extension study, there was a case of squamous cell carcinoma after 16 months with baricitinib 2 mg, and one ductal carcinoma in situ after 10 months with baricitinib 4 mg in BRAVE- AA1.
There were no VTEs, opportunistic infections, cases of tuberculosis, gastrointestinal perforations, or deaths were reported in either trial over the observation period.
Summary Points
The authors point out that patients “continued to improve” over 52 weeks. This is true but it’s a pretty small improvement from week 36 to week 52. Also, we’ve lost the placebo group so we don’t really have a good understanding of the real effects.
It’s surprising that the proportion of patients achieving a SALT 20 are not better than this. I was hoping the number would land somewhere near 50 %. Nevertheless it’s encouraging data. There are still lots of patients that don’t benefit from baricitinib as much as we’d hope.
The safety data is pretty good. There are no new safety concerns emerging. There were no blood clots in the extension trial. There were new cancers but it’s not clear is there are casual associations or not. Long term follow up data will be important.
REFERENCE
Kwon O et al. Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata over 52 Weeks of Continuous Therapy in Two Phase III Trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023 Mar 1;1-9.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.