The Phase 3 Trial Data for Baricitinib:
Phase 3 Baricitinib Data are Now In
The long awaited phase 3 baricitinib data are in.
Authors present data for two randomized, placebo-controlled, phase 3 trials (BRAVE-AA1 and BRAVE-AA2) involving adults with severe alopecia areata with a SALT score of 50 to 100 (100 means complete hair loss). Patients were randomly assigned in a 3:2:2 ratio to receive once-daily baricitinib at a dose of 4 mg, baricitinib at a dose of 2 mg, or placebo. The primary outcome was a SALT score of 20 or less at week 36.
The BRAVE -AA TRIALS
BRAVE-AA1 and BRAVE-AA2 are double-blind, parallel-group, randomized, placebo-controlled trials conducted at 169 centers in 10 countries. Patients were 18-70 years of age.
The following were the inclusion criteria for patients recruited into the study:
a) Patients with SALT Score 50 or higher
b) a current episode of alopecia areata lasting more than 6 months to less than 8 years, without spontaneous improvement (≤10-point reduction in the SALT score) during the previous 6 months.
c) Patients with episodes lasting at least 8 years were permitted to enroll only if episodes of hair regrowth, spontaneous or while receiving treatment,
d) no other hair loss conditions
e) no use of topical steroids on the scalp or eyebrows within 1 week before randomization
f) no use of systemic or intralesional glucocorticoids (including intraarticular injections) within 8 weeks before randomization,
g) no use of topical or oral JAK inhibitors within 4 or 8 weeks, respectively, before randomization.
h) Patients inadequate response to oral JAK inhibitors in last 12 weeks
Primary Outcome of the Study
The primary outcome was a SALT score of 20 or less at week 36. in research studies, this is often said to be a meaningful outcome in AA studies.
Secondary outcomes
A variety of secondary outcomes were assessed including
1) a Scalp Hair Assessment Patient-Reported Outcome (PRO) score
2) a Clinician-Reported Outcome (ClinRO) Measure for Eyebrow Hair Loss score
3) a ClinRO Measure for Eyelash Hair Loss score
4) the percent change from baseline in the SALT score at week 36
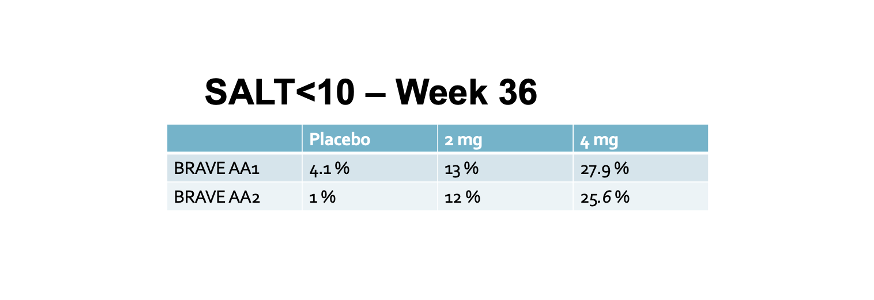
5) SALT score of 10 or less at week 36
6) a decrease (improvement) of at least 90% from baseline in the SALT score (SALT90) at week 36
7) a SALT score of 20 or less at week 24
8) a SALT score of 10 or less at week 24
9) a decrease (improvement) of at least 50% from baseline in the SALT score (SALT50) at week 12
10) a SALT score of 20 or less at week 16.
and others (including SALT 100)
Results
There were 654 patients enrolled in the BRAVE-AA1 trial and 546 patients in the BRAVE-AA2 trial. 598 (91.4%) and 490 (89.7%), respectively, completed 36 weeks of baricitinib or placebo. About 45-50 % of patients had alopecia universalis and 50-55 % had quite advanced forms. Specifically, 53.2% of the patients had very severe disease as reflected by a SALT score of 95 to 100;
The mean duration of the current episode of alopecia areata was 3.6±3.9 years in BRAVE-AA1 and 4.3±4.9 years in BRAVE-AA2. 30-40% of patients had alopecia areata for more than 4 years.
45-60 % were white; 30-40 % were Asian; 7-10 % of patients were black.
The Primary End point Results
The estimated percentage of patients with a SALT score of 20 or less at week 36 was 38.8% with 4-mg baricitinib, 22.8% with 2-mg baricitinib, and 6.2% with placebo in BRAVE-AA1 trial.
In the BRAVE -AA2 trial, the estimated percentage of patients with a SALT score of 20 or less at week 36 was 35.9% for 4 mg, 19.4% for 2 mg, and 3.3% for placebo.
Secondary Outcomes
Secondary outcomes for baricitinib at a dose of 4 mg but not at a dose of 2 mg generally favored baricitinib over placebo. 4 mg was clearly better than 2 mg.
In BRAVE-AA1, the results with 4-mg baricitinib differed significantly from those with placebo for these secondary outcomes
In BRAVE-AA2, the results with 4-mg baricitinib differed significantly from those with placebo for the first seven secondary outcomes but failed for a SALT score of 10 or less at week 24, and the subsequent two secondary outcomes did not pass statistical testing.
Secondary End Point : SALT Score Less than 10
About one quarter of patients achieved a SALT less than 10.
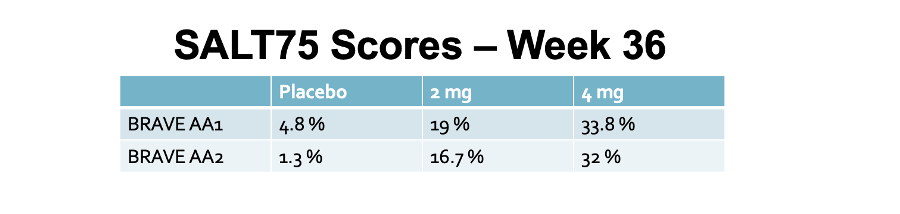
Secondary End Point: SALT 75 Scores (75 % Reduction in SALT SCORE)
In the BRAVE AA1, 25.6 % had SALT75 by week 24 using 4 mg and 33.8 % had SALT 75 by week 36. This compared to 3.7 % and 4.8 % of placebo, respectively (week 24 and 36).
In the BRAVE AA2, 26.9% had SALT 100 by week 24 and 32 % had SALT 100 by week 36. This compared to 1.3 % and 1.3 % of placebo, respectively (week 24 and 36).
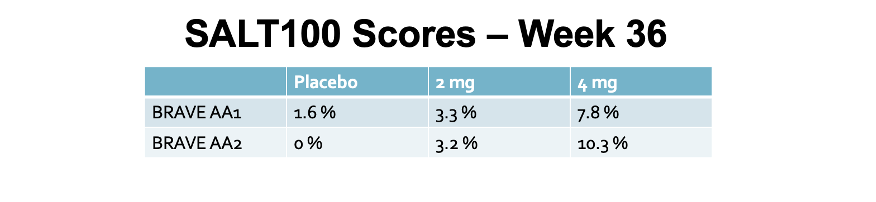
Secondary End Point: SALT 100 Scores (100 % Reduction in SALT SCORE)
In the BRAVE AA1, 5.3 % had SALT 100 by week 24 and 7.8 % had SALT 100 by week 36. This compared to 0.5 % and 1.6 % of placebo
In the BRAVE AA2, 6.0 % had SALT 100 by week 24 and 10.3 % had SALT 100 by week 36. This compared to 3.2 % and 0 % of placebo
Side Effects of Baricitinib
Side effects were generally mild to moderate in most but not all. The percentages of patients who dropped out because of adverse events were low and were similar across the trial groups.
Common adverse events in both trials were acne, upper respiratory tract infections, headache, urinary tract infection, and elevated creatine kinase levels. Herpes zoster occurred in a low proportion of patients but was slightly more common among those who received the trial drug than among those who received placebo in BRAVE-AA2. LDL was increased in about 1/4 of patients using the drug.
Serious adverse events occurred in 2.1 % with 4-mg baricitinib, 2.2 % with 2-mg baricitinib, and 1.6 % with placebo in BRAVE-AA1. In BRAVE AA2, they occurred in 3.4 % with 4 mg,2.6 % with 2 mg and 1.9 % with placebo.
In the BRAVE -AA1 study, adverse events occurred in 167 of 280 patients (59.6%) with 4-mg baricitinib, 93 of 183 (50.8%) with 2-mg baricitinib, and 97 of 189 (51.3%) with placebo.
In the BRAVE -AA2 study, adverse events occurred in 154 of 233 patients (66.1%) using 4 mg, 106 of 155 (68.4%) using 2 mg and 97 of 154 (63.0%) using placebo.
1) ACNE
Acne was more common with baricitinib than with placebo, occurring in 16 of 280 patients (5.7%) with 4-mg baricitinib, 10 of 183 (5.5%) with 2-mg baricitinib, and 1 of 189 (0.5%) with placebo in BRAVE-AA1 and in 11 of 233 patients (4.7%), 9 of 155 (5.8%), and 3 of 154 (1.9%), respectively, in BRAVE-AA2.
2) UTIs
The incidence of urinary tract infection was higher with baricitinib than with placebo in BRAVE-AA2, with such infections occurring in 11 of 233 patients (4.7%) with 4-mg baricitinib, 12 of 155 (7.7%) with 2-mg baricitinib, and 2 of 154 (1.3%) with placebo.
3) INFECTIONS
Infections were reported in 88 of 280 patients (31.4%) with 4-mg baricitinib, 46 of 183 (25.1%) with 2-mg baricitinib, and 53 of 189 (28.0%) with placebo in BRAVE-AA1 and in 69 of 233 patients (29.6%), 58 of 155 (37.4%), and 45 of 154 (29.2%), respectively, in BRAVE-AA2 (Table 3).
Herpes zoster infections — occurring in 2 of 280 patients (0.7%) with 4-mg baricitinib, 1 of 183 (0.5%) with 2-mg baricitinib, and 1 of 189 (0.5%) with placebo in BRAVE-AA1 and in 3 of 233 patients (1.3%), 3 of 155 (1.9%), and 1 of 154 (0.6%), respectively, in BRAVE-AA2. Zoster infections were localized and there were no disseminated infections.
4) BLOOD CLOTS
There were no venous thromboembolic events
5) HEART ATTACKS and CHF
In BRAVE-AA1, a myocardial infarction occurred in a patient who received 2-mg baricitinib and had cardiovascular risk factors.
One patient had congestive heart failure (CHF) in BRAVE-AA-2
6) CHOLESTEROL
Increased levels of low-density lipoprotein (LDL ) cholesterol were observed in approximately 25% of patients in the baricitinib groups compared to 15 % of placebo.
7) CK
A elevated creatine kinase level to more than 5 times the upper limit of the normal range was observed in small percentages of patients in the baricitinib groups.
6) CANCER
The study was too short to get meaninful cancer data. In BRAVE-AA2, one case of B-cell lymphoma was reported in a patient who received 4-mg baricitinib, and one case of prostate cancer was reported in a patient who received placebo.
Conclusion
Overall, an important study for us all. Baricitinib 4 mg is better than 2 mg and better than placebo. About one third of patients achieve what we would call good results. About 1/4 achieve outstanding results. It would be nice to understand the data according to alopecia totalis and universalis and it’s unfortunate this data isn’t clearly laid out and one needs to dig dig dig through data to try to get a sense of these estimates. It seems it’s probably 15% of so of really advanced AA who get good responses but again this really needed to be presented.
All papers with JAK inhibitors should be forced to directly address the FDA black box warning on cancer and heart disease with JAK inhibitors that spans the class of drugs. This was not directly addressed here. Granted, the trial is far too short for any indication of cancer risk and heart disease to really emerge with any good numbers, the topic should be bravely addressed. Editors of journals should demand at least once sentence.
REFERENCE
King B et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N Engl J Med. 2022 Mar 26.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.