3 More Cases of Scarring Alopecia with Checkpoint Inhibitors
SCARRING ALOPECIA AND THE CHECKPOINT INHIBITOR DRUGS
Immunotherapy with anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies is now a standard therapy for many malignancies
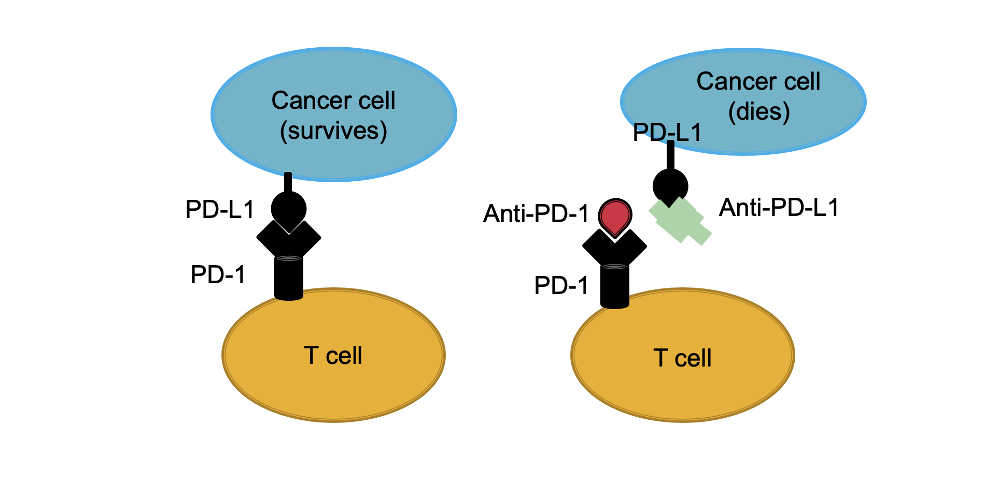
In order to understand immunotherapy, we need to understand immune checkpoints. Immune ‘checkpoints’ are a normal part of the immune system. The role of these checkpoints is to prevent an immune system response from being so strong that it destroys healthy cells inside in the body.
Proteins on the surface of T cells (called immune checkpoint proteins) can bind proteins on the surface of cancer cells (called partner proteins). When T cell checkpoint proteins engage with these partner proteins, the T cell is ultimately sent a signal to “settle down” and not proceed to kill the cell it is bound to. This is not good when it comes to cancer as it prevents the T cell from killing the cancer cell.
New drugs known as immune checkpoint inhibitors act to blocking checkpoint proteins from binding with their partner proteins. Some checkpoint inhibitors also bind to the partner protein to prevent the interaction. All in all, this prevents the T cell from being told to ‘settle down’ and the results is the T cell then proceeds to kill the cancer cell.
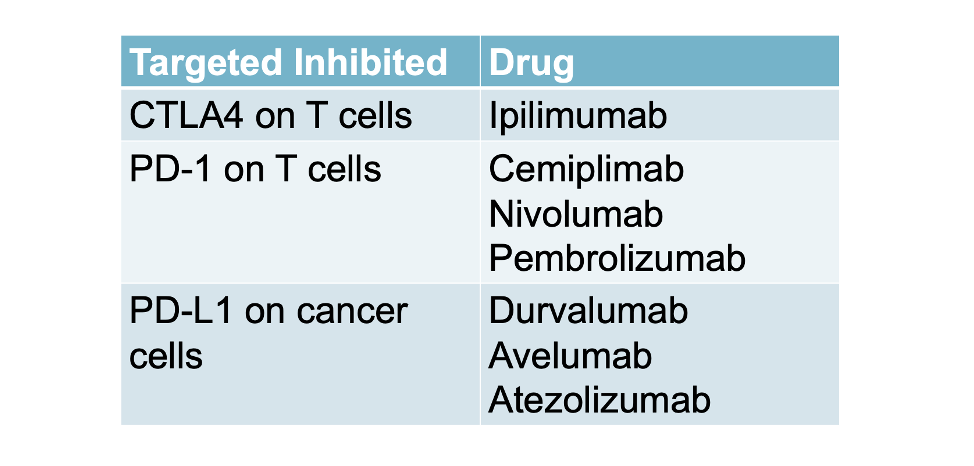
Ipilimumab: The first FDA approved checkpoint inhibitor
The first immune checkpoint inhibitor was approved by the Food and Drug Administration in 2011. It is an antibody targeting the cytotoxic T lymphocyte antigen 4 (CTLA4). The drug is known as ipilimumab and was approved in 2011 for treatment of melanoma. To date, there are numerous checkpoint inhibitor drugs approved and being studied. Some are summarized below:
Checkpoint Inhibitors Increase the Risk of Autoimmune Reactions
Immune activation may also lead to production of antibodies against self-antigens. Therefore, checkpoint inhibition can induce autoimmune responses, generating immune-related adverse events. Immune activation may also lead to production of antibodies against self-antigens. Therefore, checkpoint inhibition can induce autoimmune responses, generating immune-related adverse events.
What is known about “lichenoid” skin reaction with anti-PD-1 drugs?
Lichenoid skin reactions are common immune-related adverse skin reactions and are observed in 17.1% of patients treated with PD-1 inhibitors. A variety of lichenoid reactions are known to occur with checkpoint inhibitors. Lichen planus, hypertrophic lichen planus, lichen planus pemphigoides, lichenoid dermatitis and lichen planopilaris have all been reported in several publication after use of checkpoint inhibitors.
Prior Studies have Drawn Attention to Possible LPP with Checkpoint Inhibitors
Several prior studies have draw attention to the possibility that immune checkpoint inhibitors can cause scarring alopecia. In the Evidence Based Hair Podcast, Season 1, Episode 11, we reviewed many prior studies linking these drugs to scarring alopecia including those listed below.
Braasch et al. 2022: 3 More cases of Scarring alopecia with Checkpoint Inhibitors
A new study by Braasch et al report three more cases of scarring alopecia with checkpoint inhibitors. Here, 2 cases of lichen planopilaris (LPP) and 1 case of frontal fibrosing alopecia (FFA)
PATIENT 1: FFA-LIKE CHANGES ASSOCIATED WITH NIVOLUMAB AND IPILIMUMAB
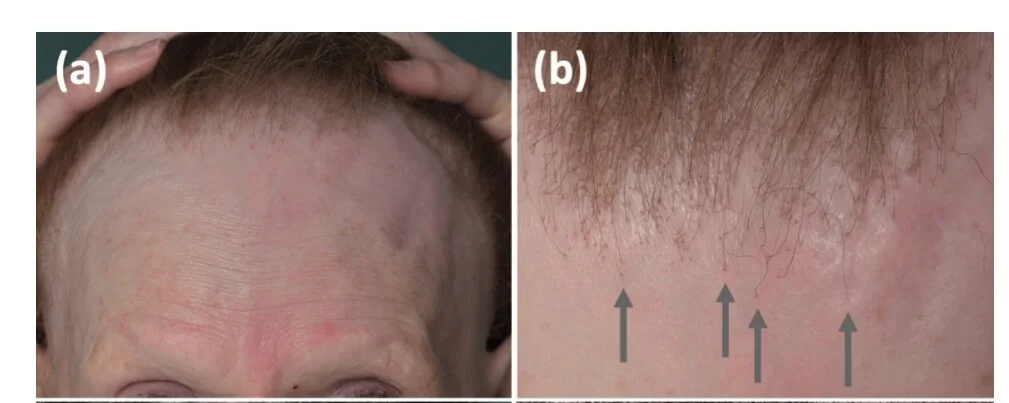
The first patient was a 62-year-old woman with pulmonary metastatic melanoma who was treated with nivolumab and ipilimumab combination therapy. She developed an FFA like clinical picture just 2.5 months after starting the drugs. There was complete hair loss of the eyebrows along with frontotemporal hairline recession. A scalp punch biopsy revealed findings consistent with LPP. She was treated with topical clobetasol but did not wish other therapies like hydroxychloroquine. Nivolumab was discontinued after 1.5 years and after 3 years she still showed continued remission of the metastatic lung disease. FFA, however, was slowly progressing .
Patient 1 with an FFA like presentation associated with use of nivolumab and ipilimumab checkpoint inhibitor drugs. It is assumed that this image is from 2.5 months after starting which would make this 5 cm hairline recession an incredibly rapid form of FFA unseen in most cases of FFA. From Braasch et al. Scarring Alopecia Under Immune Checkpoint Blockade: a Report of Three Cases. Acta Derm Venereol. 2022 Oct 11;102:adv00792. Image used under creative commons license.
PATIENT 2: LPP-LIKE CHANGES ASSOCIATED WITH PEMBROLIZUMAB
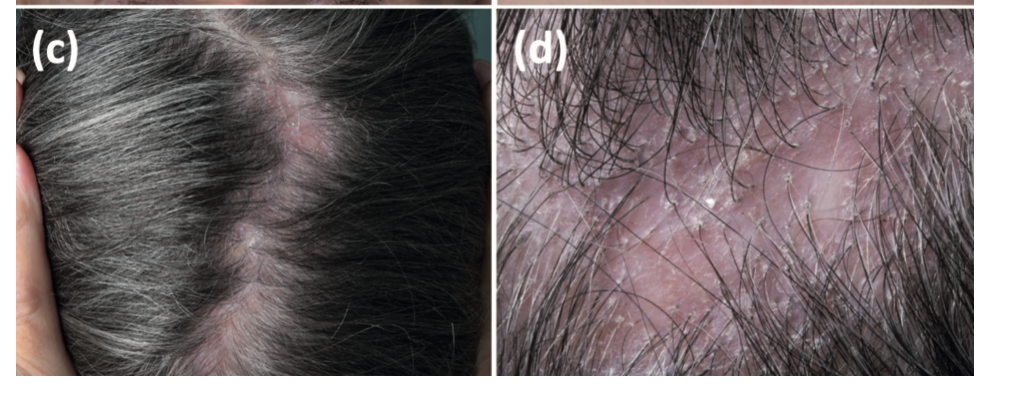
The second case is from a 76-year-old woman with incomplete-resected mucosal melanoma of the paranasal sinus who received the checkpoint inhibitor pembrolizumab for over 2 years. After 2 years of treatment she presented with a scarring alopecia. Topical clobetasol solution was started which helped reduce her inflammation. She stopped pembrolizumab after 2.5 years of treatment due to sustained complete remission.
Patient 2 with an LPP like presentation associated with use of checkpoint inhibitor drug pembrolizumab. Hair loss started after about 2 years of use of the drug. From Braasch et al. Scarring Alopecia Under Immune Checkpoint Blockade: a Report of Three Cases. Acta Derm Venereol. 2022 Oct 11;102:adv00792. Image used under creative commons license.
PATIENT 3: LPP-LIKE CHANGES ASSOCIATED WITH NIVOLUMAB
The third patient in the report was a 63-year-old woman with malignant melanoma received adjuvant therapy with nivolumab after complete resection of a single cutaneous metastasis. Following 16 weeks of therapy, she reported severe pruritus and hair loss from the scalp. A biopsy confirmed lichen planopilaris (LPP). Topical therapy with clobetasol 0.5 mg/g solution led to a rapid improvement in pruritus and she proceeded with nivolumab.
COMMENT
These are three more cases of scarring alopecia associated with use of checkpoint inhibitor drugs. Hair loss developed 2.5 months, 2 years and 16 weeks after starting the drug indicating quite a bit of variability in when hair loss actually occurs.
We need to be aware of the array of autoimmune skin reactions these drugs can cause especially the autoimmune lichenoid type reactions which can affect up to 17% of patients. The scarring alopecias are a potential side effect that we need to be able to recognize and treatment. Topical steroids, steroid injections, topical tacrolimus, doxycycline, hydroxychloroquine and retinoids are all possible treatment considerations.
MAIN REFERENCE
Braasch et al. Scarring Alopecia Under Immune Checkpoint Blockade: a Report of Three Cases. Acta Derm Venereol. 2022 Oct 11;102:adv00792.
ADDITIONAL REFERENCES
Garcia-Melendo C, et al. Extensive lichen planopilaris as exclusive lichenoid reaction secondary to pembrolizumab in a patient with metastatic melanoma. Dermatol Ther. 2022 Feb 17;e15388.
Uthayakumar AK et al .Severe progressive scarring pembrolizumab-induced lichen planopilaris in a patient with metastatic melanoma. Australas J Dermatol. 2021 Aug;62(3):403-406.
Cogen et al. Lichen planopilaris associated with pembrolizumab in a patient with metastatic melanoma. JAAD Case Rep . 2018 Jan 16;4(2):132-134.
Dominguez-Santas M et al. Avelumab-induced lichen planopilaris, a novel association. Int J Dermatol. 2021 Oct;60(10):e414-e416.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.