Lichen Planopilaris (LPP) in Patients Using The Tyrosine Kinase Inhibitor Nilotinib
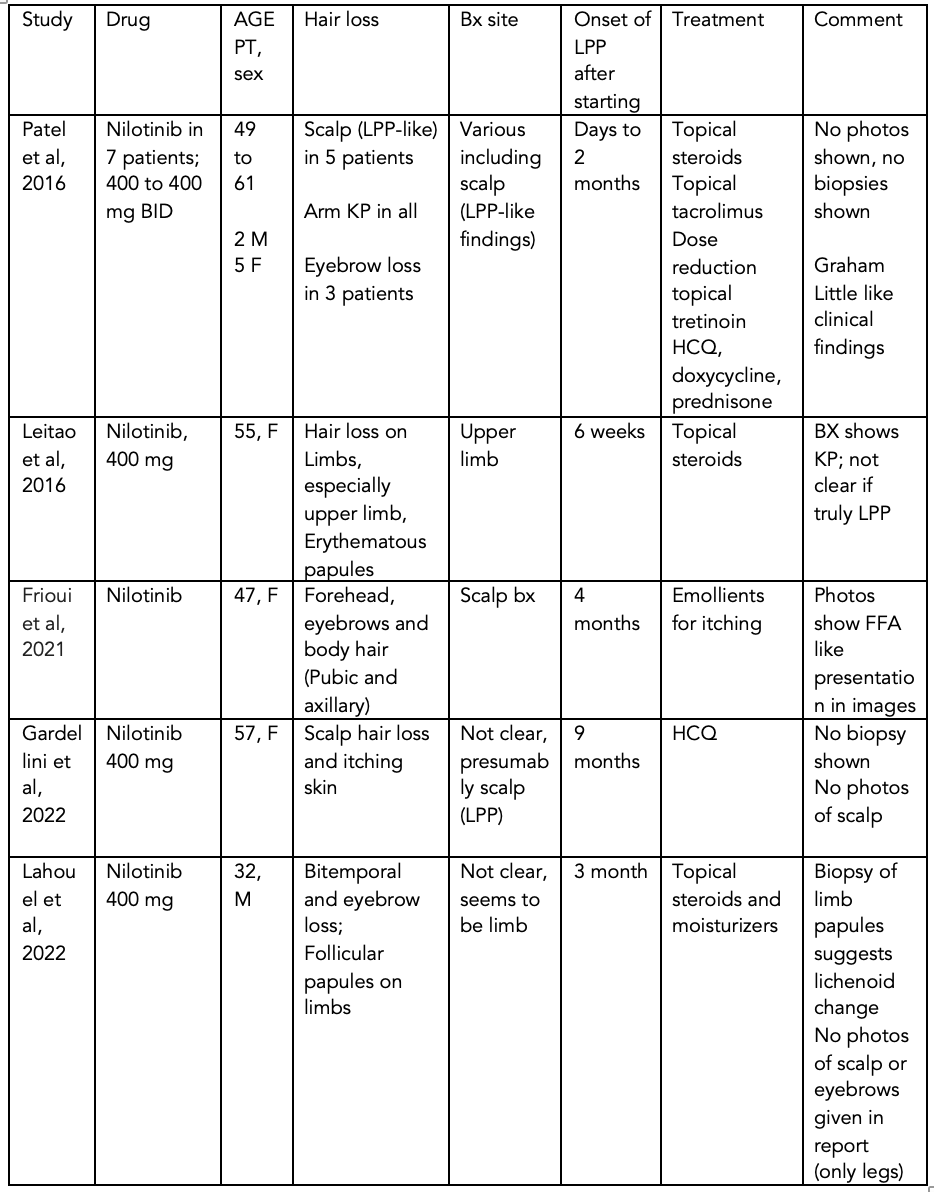
Lichen planopilaris after Nilotinib
Chronic myeloid leukemia (CML) is a type of cancer of the blood and bone marrow. The cancer is characterized by cells having a specific translocation of genetic material between chromosomes 9 and 22 resulting in the “BCR-ABL” fusion oncogene that drives the cancer.
Treatment with medications known as tyrosine kinase inhibitors (TKI) has become the mainstay of CML treatment. This has dramatically changed the prognosis for patients. In 2001, the Food and Drug Administration (FDA) approved imatinib to treat patients with CML that has the BCR – ABL translocation.
More potent second generation TKIs like nilotinib and dasatinib were approved after imatinib. Nilotinib is 20 times more potent than imatinib. Nilotinib, also known as Tasigna, was approved in 2007 for patients who are resistant to imatinib but is also approved as a first line agent as well.
The fact that Nilotinib has 20-fold higher potency is important. It not only results in the inhibition of the BCR-ABL-mediated proliferation of CML cells. This agent also inhibits the tyrosine kinases platelet-derived growth factor receptor (PDGF-R) and c-kit. It is thought that the chances of side effects may increase with greater tyrosine kinase inhibition
Two New Reports of LPP-like reaction with Nilotinib
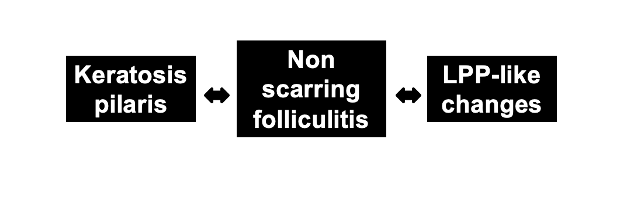
Two new reports in January support a previously held view that nilotinib may have hair follicle directed side effects which include both keratotis pilaris and lichen planopilaris.
Gardellini and colleagues, 2022
Gardellini and colleagues reported the case of a 57-year-old woman diagnosed with CML in 2018 treated with imatinib (400 mg/d) as first-line treatment. After experiencing treatment failure with imatinib, she was changed to nilotinib 800 mg per day. 9 months into treatment she developed itchy skin, hair thinning and worsening alopecia. The patient underwent scalp biopsy and the authors state that the evaluation was consistent with lichen planopilaris. The patient was treated with hydroxychloroquine at 200 mg per day and nilotinib was reduced to 600 mg per day to reduce the chances of QTc interval prolongation. She had an improvement in alopecia and mainlined her good treatment response against the cancer. She gets EKG monitoring of her heart rhythm and has not had QTc prolongation.
Lahouel and colleagues, 2022
Lahouel et al reported a case of a 32-year-old male with CML treated with nilotinib as his first line therapy (400 mg/d). The patient reportedly developed diffuse hair loss in his frontal and bitemporal scalp and a significant loss of his eyebrows. The authors state that he had follicular papules and hair loss on the trunk and extensor surface of the upper and lower limbs. A biopsy of one of the limb papules showed a lichenoid change. The authors don’t provide images of the scalp hair loss nor histology of the scalp. The histology of the limb papules seems supportive of a lichenoid folliculitis in the hair follicle – so a diagnosis of LPP seems to fit well. The patient was treated with topicial steroids and moisturizers.
Summary and Comment
It’s well known that Nilotinib can cause rashes. A meta-analysis of clinical trials evaluating Nilotinib revealed that rash occurs in 34.3% of patients, with high-grade rash present in 2.6%. The observation that tyrosine kinase inhibitors like nilotinib can cause scarring and non scarring hair loss is also not new. In fact, there are 9 prior patients reported in the medical literature that have shown these observations.
These two reports here add to the collective evidence that a lichenoid folliculitis may be a side effect of nilotinib. It seems that a range of hair follicle reactions are possible ranging from scalp LPP, body LPP, eyebrow loss, keratosis pilaris like changes on the arms, legs and abdomen. These reactions occur about 1-3 months after starting the drug if they are going to occur.
Stopping drugs is often the recommended plan whenever most drugs create a side effects. However, this is not possible with lifesaving drugs like the tyrosine kinase inhibitors which can dramatically change the survival of patients. Instead of stopping, treatment of the LPP with agents like doxycycline, hydroxychloroquine, topical steroids, topical retinoids is a possibility. The authors point out that one might need to be careful when using hydroxychloroquine with tyrosine kinase inhibitors given the potential of both to prolong the QTc interval. If one is going to use hydroxychloroquine, close EKG monitoring will be needed.
A biopsy is helpful in these cases and consultation with an expert dermatolopatholgist is helpful to help distinguish keratosis pilaris from lichen planopilaris. It seems that both morphologies are possible in patients treated with nilotinib.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.