Myophenolate Mofetil for Lichen Planopilaris: 1 in 5 Experience Complete Response
Systematic Review Highlights Benefits of Mycophenolate Mofetil.
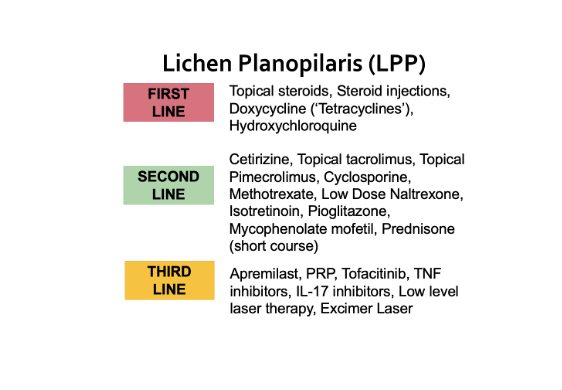
Mycophenolate mofetil is an oral immunosuppressant medication which is used for a variety of inflammatory and autoimmune conditions. It also is known by trade names such as CellCept, Myfortic. The medication was first approved in the US in 1995 to prevent graft rejection in patients undergoing kidney, liver and heart transplants.
The drug has been used off label for treating lichen planopilaris for about 15 years. It is also widely used to treat a variety of autoimmune type conditions.
Mostafa et al, 2022: 50% Partial Response; 20 % Complete Response
Mostafa et al performed systematic review and meta-analysis of all studies performed pertaining to the use of MMF for lichen planopilaris. A total of 6 studies were retrieved with data from 94 patients. Study sizes ranged from 5 to 66 patients. 20 % had complete response and partial responses were seen in 49.2 % of patients.
16.9% had side effects including increase liver enzymes (5.4%), urinary traction infections (3.1%), peripheral edema (2.3%), photosensitivity (1.5%), anemia (0.7%), hyperlipidemia (0.7%) and herpes zoster infection (0.7%).
Conclusion:
Overall, the authors concluded that MMF is an option for lichen planopilaris although it does not induce complete responses in a high proportion. It seems to be less effective than treatment options like cyclosporine but it’s reasonably good safety makes it an important option to consider. It is generally well tolerated.
I’m not personally a great fan of MMF as a “first line” option for LPP. But it is on my list of second line options and it does help a small proportion of my patients. I think the 20 % number is probably close to accurate - but may even be a tad high. Mycophenolate is certainly not a magic treatment in LPP in my opinion but can help in some cases. It’s a useful option and can be combined (with caution) for some treatment algorithms such as hydroxychloroquine. It does need monitoring for liver enzymes and a basic CBC monthly for a few months. It is an immunosuppressant and so discussion should take place with one’s physicians about a shingles vaccine before starting.
REFERENCE
Mostafa N et al. Mycophenolate mofetil and lichen planopilaris: systematic review and meta-analysis. J Dermatolog Treat. 2022 Feb;33(1):369-372.
ADDITIONAL REFERENCES (6 STUDIES OF MMF for LPP)
Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009; 28(1):3–10.
Babahosseini H, Tavakolpour S, Mahmoudi H, et al. Lichen planopilaris: retrospective study on the characteristics and treatment of 291 patients. J Dermatol Treat. 2019;30(6): 598–604.
Cho BK, Sah D, Chwalek J, et al. Efficacy and safety of mycophenolate mofetil for lichen planopilaris. J Am Acad Dermatol. 2010;62(3):393–397.
Lajevardi V, Ghodsi SZ, Goodarzi A, et al. Comparison of systemic mycophenolate mofetil with topical clobetasol in lichen planopilaris: a parallel-group, assessor- and analyst- blinded, randomized controlled trial. Am J Clin Dermatol. 2015;16(4):303–311.
Samrao A, Chew A-L, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163(6): 1296–1300.
Spencer LA, Hawryluk EB, English JC. Lichen planopilaris: retrospective study and stepwise therapeutic approach. Arch Dermatol. 2009;145(3):333–334.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.