Oral Minoxidil Fails to Beat Topical Minoxidil in Improving Hair Counts
Is oral minoxidil more effective than topical minoxidil?
A new randomized controlled study suggests that male users of 5 mg oral minoxidil once daily do not seem to get all that much more hair growth on the scalp compared to those using 5 % topical minoxidil twice daily.
As you’re probably well aware, oral minoxidil was originally used as a blood pressure medication. In fact, the FDA approved it for the treatment of hypertension back in 1979. Typical blood pressure doses were 10 to 40 mg daily. At these doses, the most common adverse effects are hypertrichosis, tachycardia, headache, and edema. It soon became clear in the early 1980s that oral minoxidil is a treatment option for males with AGA. Topical minoxidil received FDA approval for male balding just 9 years later - in 1988.
To date, there has been incredible interest in oral minoxidil. Surprisingly, despite this interest, only a few high-quality studies have been done. We discussed the Panchaprateep et al. study in the past. It wasn’t a super high-quality study, but it represented the best we had - until now.
As we’ll see now in the nicely conducted RCT by Penha et al, oral minoxidil did not seem to perform so well in males in Brazil. Specifically, it was unable to beat topical minoxidil in hair growth.
Penha et al 2024
Authors from Brazil set out to compare the efficacy, safety, and tolerability of daily oral minoxidil, 5 mg, vs twice-daily topical minoxidil, 5%, for 24 weeks in the treatment of male AGA.
This was a double-blind, placebo-controlled, randomized clinical trial conducted at a single specialized clinic in Brazil. Eligible men with AGA aged 18 to 55 years classified using the Norwood-Hamilton scale as 3V, 4V, or 5V were included and randomized. Participants were randomized 1:1 into 2 groups: oral minoxidil, 5 mg, daily and topical placebo solution; or 1 mL of topical minoxidil, 5%, twice daily and oral placebo for 24 weeks.
The oral minoxidil group received 5 mg of minoxidil capsules plus a placebo solution to apply to the scalp. The topical minoxidil group received a 5% topical minoxidil solution plus placebo capsules.
The primary outcome was the change in terminal hair density on the frontal and vertex regions of the scalp. Secondary outcomes included change in total hair density in the target area, assessment of standardized clinical photographs, and assessment of adverse effects, blood pressure, and heart rate.
Among 90 enrolled participants, 68 completed the study; of these, the mean age was about 37 years. A total of 33 participants were enrolled in the oral minoxidil group and 35 in the topical treatment group. Both groups were homogenous in terms of demographic data and AGA severity.
Most participants had mild to moderate AGA, and the groups were homogeneous. A total of 68 completed the study and had 24-week follow-up data. There were 12 dropouts (27%) in the oral minoxidil group and 10 drop outs in the topical group. There was no difference in the proportions of dropouts between groups.
When comparing oral minoxidil to topical minoxidil for the frontal area
· After 24 weeks, males using oral minoxidil had 3.1 more terminal hairs per cm2 (95% CI, −18.2 to 21.5; P = .27) and 2.6 hairs per cm2 (95% CI, −10.3 to 15.8; P = .32) for total hair density. None of this was statistically significant.
When comparing oral minoxidil to topical minoxidil for the vertex area
· After 24 weeks, males using oral minoxidil had 23.4 more terminal hairs per cm2 (95% CI, −0.3 to 43.0; P = .09) and 5.5 hairs per cm2 (95% CI, −12.5 to 23.5; P = .32) for total hair density. None of this was statistically significant.
When examining “percent differences” instead of absolute differences, the authors found some differences.
FOR TERMINAL HAIR DENSITY CHANGES In the VERTEX, the percentage increase in terminal hair density was 27.1% (95% CI, 6.5-47.8) higher for the oral minoxidil group than the topical group (P = .005). This was statistically significant.
FOR TERMINAL HAIR DENSITY CHANGES In the FRONTAL SCALP, the percentage increase in terminal hair density was 13.1% higher for the oral minoxidil group (95%CI,−11.5 to 37.5%;P = .15). However, this was not statistically significant.
FOR TOTAL HAIR DENSITY IN THE VERTEX, there was an increase of 2.1% (95% CI, −8.1 to 12.3; P = .27) in the oral minoxidil group compared with the topical minoxidil group. This was not a statistically significant difference
FOR TOTAL HAIR DENSITY IN THE FRONTAL SCALP, there was a decrease of 0.2% (95% CI, −8.4 to 8.0; P = .89) ) in the oral minoxidil group compared with the topical minoxidil group. This was not a statistically significant difference.
PHOTOGRAPHIC ANALYSIS
According to the photographic analysis of the 3 dermatologists blinded to treatments, oral minoxidil was superior to topical minoxidil on the vertex (24%; 95% CI, 0 to 48; P = .04) but not on the frontal scalp (12%; 95% CI, −12 to 36; P = .24).
Specifically, 60% of males in the oral minoxidil group (60%) and 48% topical minoxidil group (48%) were rated to have a clinical improvement in the frontal area, with no significant difference between the groups (difference, 12%; 95% CI, −12 to 36;P = .24). For the vertex, 70% of patients in the oral minoxidil were rated to have a clinical improvement in the vertex area compared to 46 % in the topical minoxidil group (16 of 35 [46%]) (difference, 24%; 95% CI, 0-48; P = .04). This was statistically significant.
SIDE EFFECTS
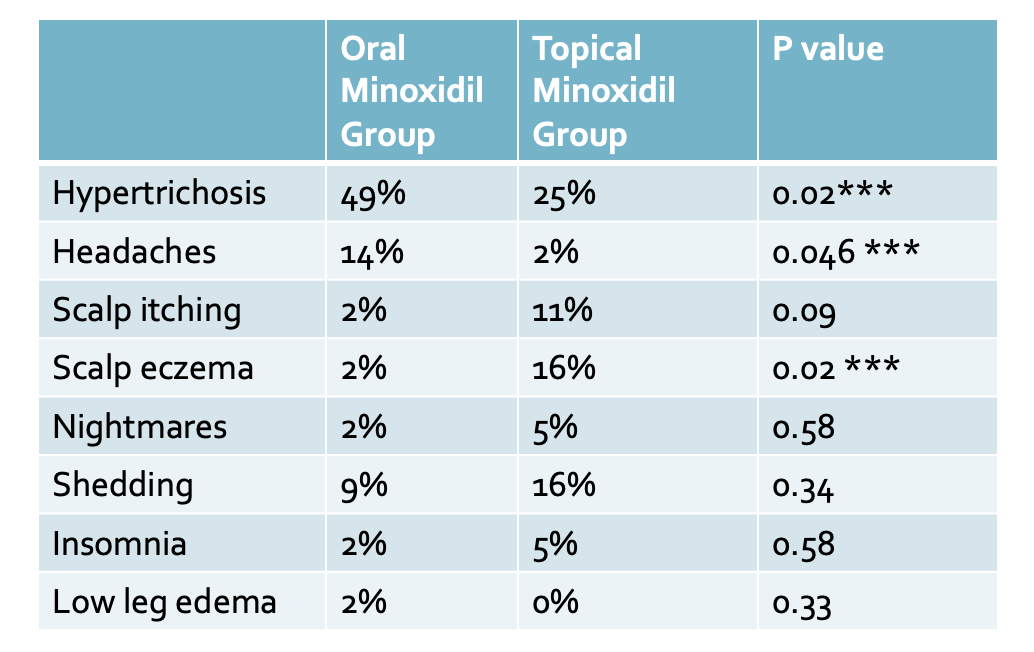
The most common adverse effects in the oral minoxidil group were hypertrichosis (22 of 45 [49%]) and headache (6 of 45 [14%]). The most common side effect in the topical minoxidil group was hypertrichosis present in 25 % followed by scalp eczema in 16 % of users. There was no difference between the groups regarding variation in mean arterial blood pressure over time. Transient hair loss in the first two months was experienced by treatment by 9% in the oral minoxidil group and 16% in the topical minoxidil group, but this was not statistically significantly different.
COMMENT
This is an important study for the history books. First, it is the first well done comparative therapeutic trials of low-dose oral minoxidil for male AGA.
The study showed that oral minoxidil, 5 mg, once per day for 24 weeks did not demonstrate superiority over topical minoxidil, 5%, twice per day in men with AGA – at least for hair growth and the primary end point of actual numbers.
Despite these data, there is a hint that oral minoxidil is better for the crown in males. Expert photographic evaluation at the vertex and the percent increase of terminal hair density in the vertex area suggests that oral minoxidil probably beats out topical minoxidil.
Hypertrichosis was the main adverse effect reported and was more prevalent in the oral minoxidil group. Headache was more prevalent in the oral minoxidil group. Transient hair loss in the first two months was experienced by treatment by 9% in the oral minoxidil group and 16% in the topical minoxidil groups.
This study is a nice reminder of the true power of randomized trials. This study gave far inferior results for oral minoxidil than the much loved 2020 study by Panchaprateep et al. in the 2020 study, 93 % of oral minoxidil users had nice results compared to 69 % here in the Penha et al study. Further more, there was not a single user in the Panchaprateep et al. who failed to benefit from oral minoxidil. In contrast, about 1/3 of patients in the Penha et al study experienced no improvement or actually to worse.
The debate on exactly how well oral minoxidil works is far from over!
REFERENCE
Mariana Alvares Penha 1, Hélio Amante Miot 1, Michal Kasprzak 2, Paulo Müller Ramos. Oral Minoxidil vs Topical Minoxidil for Male Androgenetic Alopecia: A Randomized Clinical Trial. JAMA Dermatol . 2024 Apr 10:e240284. doi: 10.1001/jamadermatol.2024.0284. Online ahead of print
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.