New Data Supports Benefits of Ritlecitinib in Adolescents (12-17)
30-50 % of Adolescents with Advanced Alopecia Areata Experience Significant Regrowth with Ritlecitinib (Litfulo)
On June 23, 2023, Ritlecitinib (LITFULO) was FDA approved at 50 mg once daily for treating advanced alopecia areata in patients 12 and older.
It was the ALLEGRO 2b/3 trials that ultimately led to the FDA approval of ritlecitinib. We reviewed the ALLEGRO trial back in June on a webinar.
Overall about 23 % of patients receiving 50 mg achieved a SALT <20 (fairly good regrowth) and at week 24 and 43 % achieved a SALT <20 at week 48.
Horinsky et al 2023: A study of Litfulo in Adolescents
A new study by Dr Hordinsky and colleagues sought to examine the ALLEGRO 2b/3 trial data as in pertains specifically to adolescents. There were 18 patients who received 50 mg ritlecitinib.
At a 50 mg dose, the proportion of adolescents with a SALT score less than 20 (fairly good regrowth) at week 48 was around 50%
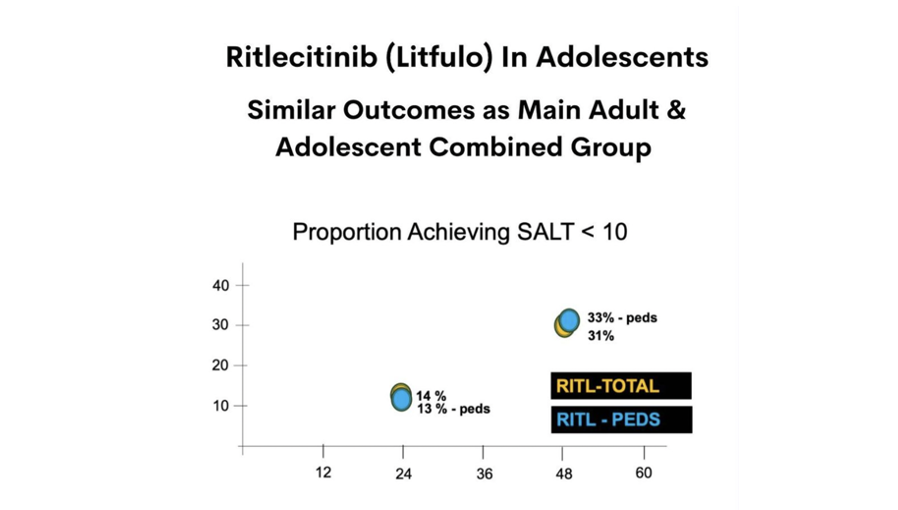
At a 50 mg dose, the proportion of adolescents with a SALT score leas than 10 (really good regrowth) at week 48 was around 33%.
These numbers and proportions shown above are somewhat similar to what was found in the “combined” adult and adolescent main study.
Side Effects of Ritlecitinib in Adolescents
The most common side effects of ritlecitinib were headaches, acne and nasopharyngitis. 2 adolescents in the main study stopped the study. One in the 50 mg group stopped due to urticaria and 1 in the ritlecitinib 10 mg stopped due to eczema. Across the entire study serious AEs were reported in 3 adolescents: one patient had appendicitis (200/30 mg group), one patient had suicidal behavior (10 mg group), and one patient had eczema (10 mg group). There were no deaths, malignancies, major adverse cardiovascular events, pulmonary embolisms, opportunistic infections, or herpes zoster infections were reported in this adolescent age group.
All in all, ritlecitinib at 50 mg is now approved for adolescents & adults with advanced alopecia areata (more than 50 % loss) in the US. Data from these 18 patients allows us to give estimates of regrowth potential: about one half will have some fairly significant regrowth and about one quarter to one third will regrow almost completely.
What happens after being on the drug 2, 5 or 50 years is not known. Patients with advanced AA generally need the drug in a continuous manner.
Reference
Hordinsky M et al. Pediatr Dermatol. 2023 Jul 17
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.