Tofacitinib: 10 Key Points from the ORAL SURVEILLANCE Trial for Hair Loss Specialists
Data from the “ORAL Surveillance Trial”: What does it mean for the alopecia community?
A new study published in the New England Journal of Medicine is important for anyone who prescribes JAK inhibitors to know very well. JAK inhibitors have come to have an important role for treating alopecia areata and have benefit in scarring alopecias such as lichen planopilaris (LPP), frontal fibrosing alopecia (FFA) and folliculitis decalvans (FD). Tofacitinib is the best studied of the JAK inhibitors for treating alopecia areata.
Although we are slowly getting long term studies of patients who use these drugs for treating rheumatoid arthritis, we actually don’t have large long term studies with these drugs in alopecia areata. All the information we get on long term risks comes from good studies of rheumatoid arthritis patients who have been using these drugs over the past decade.
Tofacitinib Use in Patients with Rheumatoid Arthritis May Increase Risk of Cancer and Possibly Cardiac Events Compared to TNF Inhibitor Therapies
Tofacitinib was FDA approved in 2012 for the treatment of more challenging cases of rheumatoid arthritis. After it was approved, the FDA required the manufacturer to continue to follow patients longer term and see how they do and see what kind of side effects they might get with longer term follow up. The FDA wanted to see ongoing safety trial comparing tofacitinib with other treatments for rheumatoid arthritis like drugs called “TNF inhibitors.”
The Oral Surveillance Study is the name of the study that meets these requirements set out by the FDA to follow patients long term and was carried out between 2014 and 2020.
It had an unusual “non-inferiority” design which is something we don’t see used in studies all that often.
The Long Awaited Oral Rheumatoid Arthritis Trial (ORAL) Surveillance trial
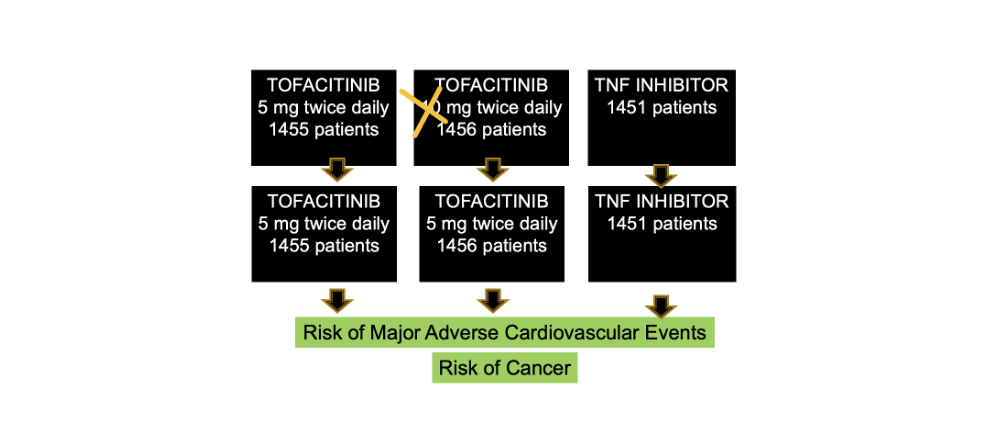
The Oral Rheumatoid Arthritis Trial (ORAL) Surveillance trial was a randomized trial evaluating the safety and efficacy of tofacitinib as compared with a TNF inhibitor in patients with rheumatoid arthritis who were 50 years of age or older and had at least one additional cardiovascular risk factor (such as current cigarette smoker, hypertension, low HDL cholesterol, diabetes mellitus, family history of premature coronary heart disease, extraarticular rheumatoid arthritis, or history of coronary artery disease). Patients had generally been tried on methotrexate first for their rheumatoid arthritis and didn’t get better.
Patients were in three groups including tofacitinib 5 mg twice daily or 10 mg twice daily or a subcutaneous TNF inhibitor (adalimumab in North America or etanercept in the rest of the world). Methotrexate was generally continued. In total, 1455 patients in the trial received tofacitinib at a dose of 5 mg twice daily, 1456 received tofacitinib at a dose of 10 mg twice daily, and 1451 received a TNF inhibitor. The median follow-up was 4 years.
In Feb 2019, patients in the 10 mg twice daily group were all switched to 5 mg twice daily after the data and safety monitoring board noted a higher frequency of pulmonary embolism (blood clots in the lung) and death from any cause among patients receiving tofacitinib at a dose of 10 mg twice daily than among those receiving a TNF inhibitor.
The primary end points of the study were to evaluate the risk of cancer and MACE (death from cardiovascular causes, non- fatal myocardial infarction, or nonfatal stroke). There were many secondary endpoints including risks of infection, and blood clots.
Results of the Oral Surveillance Trial
Results of the ORAL Surveillance study showed that tofacitinib has significantly increased risk of cancer compared to TNF inhibitor users and non-significant increases in cardiac events. Let’s look at these in turn.
a) A closer look at major cardiac events (MACE) in the ORAL Surveillance Trial
The researcher showed that patients using tofacitinib had more cardiovascular events than those using TNF inhibitors - but these differences did not seem to be significant. Nevertheless, using the strict criteria the authors chose, the authors could not prove that tofacitinib really carried the same risk as TNF inhibitors (non inferiority)
During a median follow-up of 4.0 years, the incidence of MACE was higher with the combined tofacitinib doses (3.4%; 98 patients) than with a TNF inhibitor (2.5%; 37 patients).
For patients using tofacitinib 5 mg twice daily, the overall trial results showed non-significant increased incidence rates for MACE in tofacitinib users versus TNFI users with hazard ratio 1.24 and confidence interval [0.81-1.91].
In comparing between the 5 mg and 10 mg twice daily tofacitinib doses, researchers showed similar risks of MACE.
a) A closer look at cancer risk in the ORAL Surveillance Trial
During a median follow-up of 4.0 years, the incidence of cancers (excluding nonmelanoma skin cancer) was higher with the combined tofacitinib doses (4.2%; 122 patients) than with a TNF inhibitor (2.9%; 42 patients). Hazard ratios were 1.48 (95% CI, 1.04 to 2.09) for cancers suggesting that cancers increased 48% in tofacitinib users compared to TNF inhibitors.
In comparisons between tofacitinib doses, cancer risks did not appear to be different in users of the 5 mg twice daily dose compared to the 10 mg twice daily dose. The most common cancers were lung cancer with tofacitinib and breast cancer with a TNF inhibitor.
Hazard Ratios
Overall, the hazard ratios were 1.33 (with 95% confidence interval [CI], 0.91 to 1.94) for MACE comparing all tofacitinib doses for 5 mg dosing. The confidence interval crossed zero meaning there does remain a possibility of a non signifcant trend
Hazard ratios were 1.48 (95% CI, 1.04 to 2.09) for cancers suggesting that cancers increased 48% in tofacitinib users compared to TNF inhibitors.
Number needed to Harm Data
Looking at the date another way, the researchers estimated that
a) during 5 years of treatment, 113 patients would need to be treated with tofacitinib at a dose of 5 mg twice daily rather than with a TNF inhibitor to result in one additional MACE
b) during 5 years of treatment, 55 patients would need to be treated with tofacitinib at a dose of 5 mg twice daily rather than with a TNF inhibitor to result in one additional cancer, respectively. During 10 years of treatment, 27 patients would need to be treated with tofacitinib at a dose of 5 mg twice daily rather than with a TNF inhibitor to result in one additional cancer, respectively.
Secondary End Points
In addition, when examining their secondary study endpoints, the authors showed that the incidences of opportunistic infections, herpes zoster and nonmelanoma skin cancer were higher with tofacitinib than with a TNF inhibitor. Efficacy was similar in all three groups, with improvements from month 2 that were sustained through trial completion.
The efficacy of TNF inhibitors and tofacitinib was similar in this study in all three groups.
CONCLUSIONS and SUMMARY: Top 10 Points for Hair Specialists
This study teaches us that compared to TNF inhibitors, older patients with rheumatoid arthritis who have at least one cardiovascular risk factor and use tofacitinib are at higher risk for developing cancer and possibly have a cardiovascular event too.
It is important for all of us to take note that the results here apply to rheumatoid arthritis patients who are 50 older. This study did not have a control group (such as patients who just received placebo). So we are comparing everything against TNF inhibitors as the comparison group.
It is simply not possible to extrapolate the data to someone 21 years of age with alopecia areata who is starting tofacitinib or someone 17 or someone 45. It doesn’t really even apply to someone 77 years old with alopecia areata. The Oral Surveillance study applies to those 50 and over with rheumatoid arthritis with baseline cardiovascular risk. In fact, about 48 % of patients in the study were smokers
In this paper, the authors of the paper do not set out to interpret the data any further for clinicians. This is not a ‘how to’ of tofacitinib or ‘what to do next’ with this data. The authors simply state the data and leave the rest for clinicians.
10 KEY POINTS FOR HAIR SPECIALISTS TO REMEMBER WHEN REVIEWING THE ORAL SURVEILLANCE STUDY
1. Patients with rheumatoid arthritis are at higher risk for major cardiovascular events than are persons in the general population.
Patients with rheumatoid arthritis have different background risk than patients with alopecia areata or scarring alopecia.
2. TNF inhibitors appear to decrease the risk of cardiovascular events (possibly by reducing inflammation) in patients with rheumatoid arthritis. We don’t know if the risk of MACE with tofacitinib is just a risk above TNF inhibitors or is it a true risk above someone’s baseline risk. It’s not clear, but the main risk for cardiovascular events is age.
It seems that there is a trend for tofacitinib to increase the risk of cardiovascular events compared to TNF inhibitors. As we are thinking about this we need to keep in mind that TNF inhibitors themselves actually reduce the chances of cardiovascular events. So it could be that tofacitinib and JAKs don’t really increase cardiovascular risk all that much - it’s just that they don’t help decrease it.
3. Patients with rheumatoid arthritis are generally thought to be at higher risk for cancer than are persons in the general population.
The risk of cancer in patients with RA is different than the risk of cancer in our alopecia patients. It could be that tofacitinib is fueling the development of cancer in patients at higher risk than we see in our alopecia patients. These studies need to be done.
4. The cancer risks with tofacitinib were a surprise to many but suggests that in older individuals using methotrexate, tofacitinib may increase the risk of cancer.
Many clinicians found that data on cancer risks with tofacitinib quite surprising. We need to remember that this data simply tells us that patients over 50 with rheumatoid arthritis who used methotrexate may be at increased risk for developing cancer. It does not tell us anything about cancer risk in patients with alopecia.
5. TNF inhibitors do not increase the risk of most cancers (besides NMSC and melanoma) so any true increase in cancer risk with tofacitinib likely indicates a rise above someone's baseline risk.
It’s important to take note that tofacitinib may increase the risk of cancer in patients with RA. This means that someone with RA battling cancer now or someone at increased risk for developing cancer might not want to stay on a JAK inhibitor or get started on a JAK inhibitors. Like everything, this needs to be handled on a case by case basis but the cancer risks with tofacitinib seem significant.
But is the thinking any different for those with alopecia areata? Can a patient with alopecia areata who is 23 with a strong family history of cancer get started on tofacitinib? We don’t know these answers.
6. These data are a cause for concern for how rheumatologists treat rheumatoid arthritis. Whether they are a cause for concern in other patient groups is unclear - and debated. This data applies to rheumatoid arthritis patients and any extrapolation is impossible.
As mentioned above, it is important for all of us to take note that the results of this study here apply to rheumatoid arthritis patients who are 50 and older. This study did not have a control group (such as patients who just received placebo). So we are comparing everything against TNF inhibitors as the comparison group.
It is simply not possible to extrapolate the data to someone 21 years of age with alopecia areata who is starting tofacitinib or someone 17 or someone 45. It doesn’t really even apply to someone 77 years old with alopecia areata. The Oral Surveillance study applies to those 50 and over with rheumatoid arthritis with baseline cardiovascular risk.
7. We don’t know if these risks apply to all JAK inhibitors.
The FDA included other JAK inhibitors in the “black box warning” as there is no compelling evidence to show that the results are not a group effect of JAK inhibitors and “only” applicable to tofacitinib. Right now, the FDA considers the increased adverse event rates to be a class effect for all JAK inhibitors.
The FDA feels the data is significant enough that they have changed how tofacitinib is prescribed.
The FDA feels the data is significant enough that they have changed how tofacitinib is prescribed to rheumatoid RA patients.In patients with rheumatoid arthritis who have an incomplete response to methotrexate and have active disease, a TNF inhibitor will be preferred to tofacitinib for a new start, especially in persons 65 years of age or older.
8. These data don’t mean that JAK inhibitors can’t be used in RA nor do they mean we can’t use them in alopecia areata.
The only thing we can say right now is that for a patient over 50 with a diagnosis of rheumatoid arthritis who has cardiovascular risk factors and who failed methotrexate, a TNF inhibitor is probably the next best step in treatment rather than a JAK inhibitor.
We can’t extrapolate anything after patients with alopecia areata as this study did not include alopecia areata. This was a study of rheumatoid arthritis.
9. Shared decision making is key to so much in medicine and certainly that’s true for counselling about tofacitinib.
There are likely going to be some patients with RA for which starting a JAK inhibitor will be the right treatment plan. But other options need to be considered too and the risk and benefits carefully weighed.
For advanced alopecia areata (alopecia totalis and universalis), the discussion is different than in RA because we do not have many good options for refractory disease. The options for treatment for advanced AA are much narrower than for RA and other autoimmune diseases.
10. We need these long term studies in alopecia areata.
We can’t extrapolate much of the information in the oral surveillance study to a young healthy patient with alopecia areata. It’s simply guessing to say that tofacitinib will increase the risk of heart disease in these patients. It’s simply guessing to say that it will increase the risk of cancer.
We need good long term surveillance studies in alopecia areata to really understand the long term risks with use of a JAK inhibitor.
REFERENCE
Ytterberg SR et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022 Jan 27;386(4):316-326. doi: 10.1056/NEJMoa2109927.PMID: 35081280 Clinical Trial.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.