Tofacitinib, Blood Clots and Alopecia Areata: What sort of things should we be thinking about?
Reconsidering the Risk of Blood Clots in Alopecia Areata Patients Using Tofacitinib (Xeljanz)
Tofacitinib is increasing used ‘off label’ in treating alopecia areata. New data suggests that ‘standard’ doses of tofacitinib (Xeljanz) may be associated with an increased risk of blood clots in the venous system. This articles summarizes this information and reviews important considerations for patents with alopecia areata.
We learned in early 2019 that high dose tofacitinib (i.e. higher than we typically use in alopecia areata) was associated with an increased risk of patients developing blood clots.
READ: High dose tofacitinib for Alopecia Areata: What do we need to Tell our Patients?
This information came from a study known as “ A392133” which is conducted by the Pfizer company. This particular study is very important because it allows surveillance of potential side effects in the years after the drug was launched to the public. Study A3921133 was designed to help evaluate the safety of tofacitinib at two doses (5 mg twice daily and 10 mg twice daily) and compare side effects to patients using a tumor necrosis factor inhibitor (TNFi). This study was designed to assess the risk of cardiovascular (CV) events. In this particular study, patients were required to be at least 50 years of age and have at least one CV risk factor to be eligible for participation in this study. All patients entered the study on stable doses of the immunosuppressive drug methotrexate.
The data safety monitoring board that oversees that study observed that patients treated with tofacitinib 10 mg twice daily had a statistically and clinically important increase in the occurrence of pulmonary embolism, compared with patients in this study who were treated with a TNFi. The DSMB also noted an increase in overall mortality in the 10 mg twice daily treatment group compared to the tofacitinib 5 mg twice daily and TNFi treatment arms. Back in early 2019, the drug safety board did not quite have enough data ready to determine if the 5 mg twice daily dose was associated with an increased risk of blood clots. However, the data for the 10 mg twice daily does was clear and they announced their findings and recommendations to the world.
As a result of this study, Pfizer took steps to move rheumatoid arthritis study patients who were on tofacitinib 10 mg twice daily down to tofacitinib 5 mg twice daily.
What changed after the ‘early 2019’ warnings?
It wasn’t clear after the these warnings came out whether patients with alopecia areata would be any different than patients with rheumatoid arthritis. In general, patients with alopecia areata are often healthier as a general group than RA patients. After the new warnings of a chance of blood clots on the high doses of tofacitinib many physicians who treat alopecia areata with JAK inhibitors chose to limit how high they increase the doses in their patients. Myself included. The new data warned us all that going up to 10 mg twice daily potentially brings new risk to the patient.
New data suggests that even the 5 mg twice daily dosing could be associated with a small risk of blood clots
New data from the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) concluded last week that tofacitinib (Xeljanz, Pfizer) could also increase the risk of pulmonary embolism (PE) and deep vein thrombosis (DVT) in patients using the 5 mg twice daily dose - in addition to the 10 mg twice daily dose that we all learned earlier. The committee proposed that new warnings be considered for users of tofacitinib. As a result is is expect that the product information for tofacitinib will be updated with new warnings and recommendations based on data from the study and will list blood clots as an uncommon side effect occurring in between 1 in 1000 and 1 in 100 patients. The data comes after the PRAC completed its entire review of the data of the A392133 trial.
We still don’t know quite how and if this information applies to patients with alopecia areata. Do patients with alopecia areata using 5 mg twice daily have an increase risk of blood clots similar to rheumatoid arthritis patients? That we don’t know.
Blood clots: A Brief Review
I think with this new data, however, it’s important for hair specialists to go one step further than simply committing to memory that tofacitinib might increase the risk of blood clots. I think it’s important for physicians prescribing tofacitinib to remind themselves of the risk factors for blood clots and to consider which of their patients might, in fact, be at the highest risk. Patients deemed at higher risk for blood clots might best avoid using this drug - at least for now.
When we talk about blood clots in this case, we’re talking about two types of blood clots. These are blood clots in the veins of the legs (called deep vein thromboses, or “DVTs”) and blood clots that travel from the legs up into the lungs (called pulmonary embolism, or “PE”). Together, we refer to deep vein thrombosis and pulmonary embolism as venous thrombosis, or venous thromboembolism (VTE).
How common in is VTE?
VTE occurs every year in 1 in 1000 adults. About two-thirds of all VTE events are deep vein thromboses (DVTs) and one-third are pulmonary emboli (PEs)i. After age 45, the rates risk quite sharply. VTE is much less common in those in their 20s and 30’s. Men are slightly more affected than women. Studies over the years have documented a long list of “risk factors” that increase the chance for someone to develop VTE. These so called risk factors include:
Surgery
Hospitalization
Obesity
Pregnancy
Immobility (including prolonged inactivity, or long trips in the car or by plane)
Smoking
Oral contraceptives and hormone use
Certain cancers
Trauma and injury
Age (especially increased risk for people over age 60)
A family history of blood clots
Previous blood clots the patient had
VTE can be quite serious in some cases, and even deadly. Outcomes of VTE include death, or having additional VTE events in the future, or having post thrombotic syndrome or having a number of bleeding problems due to the anticoagulant medications that patients need to take once they get these sorts of blood clots.
Factoring in VTE Risk in tofacitinib users
In the past, we haven’t really thought all that much about the concept of VTE when prescribing tofacitinib in the setting of alopecia areata. It seemed that provided we don’t go up to the 20 mg dosing (10 mg twice daily) the risk of blood clots was probably not going to be an issue. Things are a bit different now with the new EMA data - at least they could be. It seems that we at least have to give thought to the concept of VTE in users of tofacitinib even at 5 mg twice daily dosing.
Understanding all the risk factors for VTE is important. We need to be asking ourselves if the patient in front of us has a normal level of risk based on what we understand today or whether the patient could in fact have a higher than normal level of risk for a blood clot.
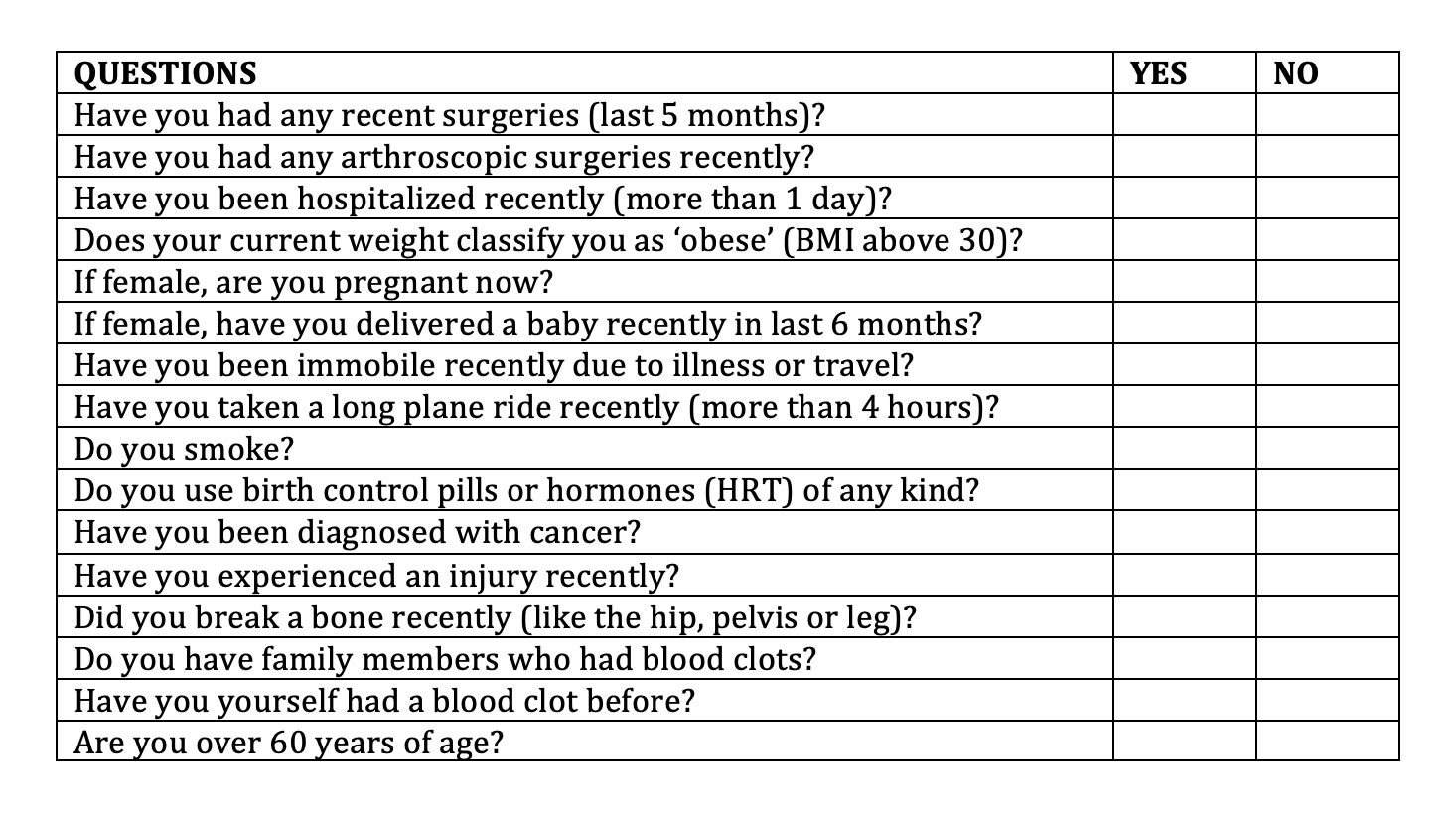
Questions to Consider Asking Patients Starting Tofacitinib
FINAL SUMMARY: Why does this matter?
We still don’t know if tofacitinib is associated with an increase risk of blood clots in patients with alopecia areata. For patients with rheumatoid arthritis who are a bit older, it appears that the 5 mg twice daily and the 10 mg twice daily dose are both associated with an increased risk for a VTE event (ie. a DVT or PE). Certainly, we must not forget that the risk with 10 mg twice daily seems much higher than 5 mg twice daily.
I think that the table above is helpful to consider when prescribing tofacitinib. We need to give careful thought to any patient who answers “yes” to any of these questions. It might not mean they can’t use tofacitinib - but it means we need to have some good discussions. The risk of blood clots is going to be quite low in a 20 year old university student who is active and healthy and is starting tofacitinib (scenario 1). But what about if we change the scenario and we consider a 20 year old female who is on an oral contraceptive and is starting tofacitinib (scenario 2)? Well, the risk of a blood clot could be higher given that oral contraceptives raise the risk of VTE. And what about a 20 year old female who is obese and uses oral contraceptives and has a mother who died of a pulmonary embolism (scenario 3). I would probably not use tofacitinib for the patient in scenario 3. The decisions in scenario 2 are made on a case by case basis but certainly there is a chance that the risk of VTE is very slightly increased. Whether the risk of VTE in a combined oral contraceptive/tofacitinib user is increased all that much above what the risk is in an oral contraceptive user alone is still not clear.
Decisions on oral contraceptive use in female patients starting tofacitinib must be addressed on a case by case basis. Preventing pregnancy is important if that is a goal of the patient herself. But reducing the risk of VTE may be another important goal. A discussion of multiple methods of contraception must be discussed.
At one time, it did seem that use of various methods of birth control and contraception was important in all female users (and possibly male users) of tofacitinib. New data, although still quite preliminary has suggested that even though tofacitinib is associated with birth defects in animal models, this might not be the case in humans using this drug. This data is still preliminary and women using tofacitinib for alopecia areata should not aim to become pregnant at this time. However, future studies may show more definite data on its safety in pregnancy - and whether women who stop Tofacitinib after they determine that they are pregnant ultimately go on to have healthy babies.
However, readers should be aware of studies of tofacitinib in pregnancy in patients with ulcerative colitis, where 11 cases of maternal exposure and 14 cases of paternal exposure have been identified across intervention studies. The outcomes include 15 healthy babies. There were with no neonatal or fetal deaths, no congenital malformations. There were two spontaneous abortions and two medical terminations. Clearly more studies are needed but this data is encouraging that tofacitinib could potentially be much less harmful in pregnancy in humans than various animal models.
References
VTE RISK WITH TOFACITINIB:
1. Xeljanx. https://www.ema.europa.eu/en/medicines/human/referrals/xeljanz
PREGNANCY OUTCOMES IN TOFACITINIB USERS
2. https://www.eurekalert.org/pub_releases/2019-11/acor-bet103119.php
3. Mahadeven U et al. Outcomes of pregnancies with maternal/paternal exposure in the Tofacitinib Safety Databases for Ulcerative Colitis.Inflamm Bowel Dis2018;24:2494–500.doi:10.1093/ibd/izy160
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.