Topical 1 % Spironolactone in the Treatment of Androgenetic Alopecia
Combination of Topical 1% Spironolactone & 5 % Minoxidil Proved Helpful in Treatment of AGA
Topical minoxidil is FDA approved for treating androgenetic alopecia in males and females. Finasteride is an antiandrogen which is FDA approved for males with AGA and the drug is also sometimes used off label in treating AGA in post menopausal women and in premenopausal women who are not considering pregnancy. A variety of other antiandrogens are used off label in treating AGA including dutasteride (males and females), spironolactone (females) and bicalutamide (females).
The oral antiandrogen spironolactone is commonly used in the treatment of androgenetic alopecia (also known as female pattern hair loss) in women. Oral spironolactone is not commonly used in males due to concerns about gynecomastia (enlargement of breast tissue in males) as well as other side effects.
New data suggests that topical spironolactone may be safe and beneficial for both males and females with androgenetic alopecia.
A New Study Comparing 5% Minoxidil vs 1 % Topical Spironolactone vs Combined Treatment
A new study by Raouf and colleagues from Egypt set out to compare the benefits of 5 % topical minoxidil gel, 1 % topical spironolactone gel and a combined 5% minoxidil/1 % spironolactone gel. The medications were compounded in 5% ethyl alcohol and 1 % hydroxy methyl propyl cellulose.
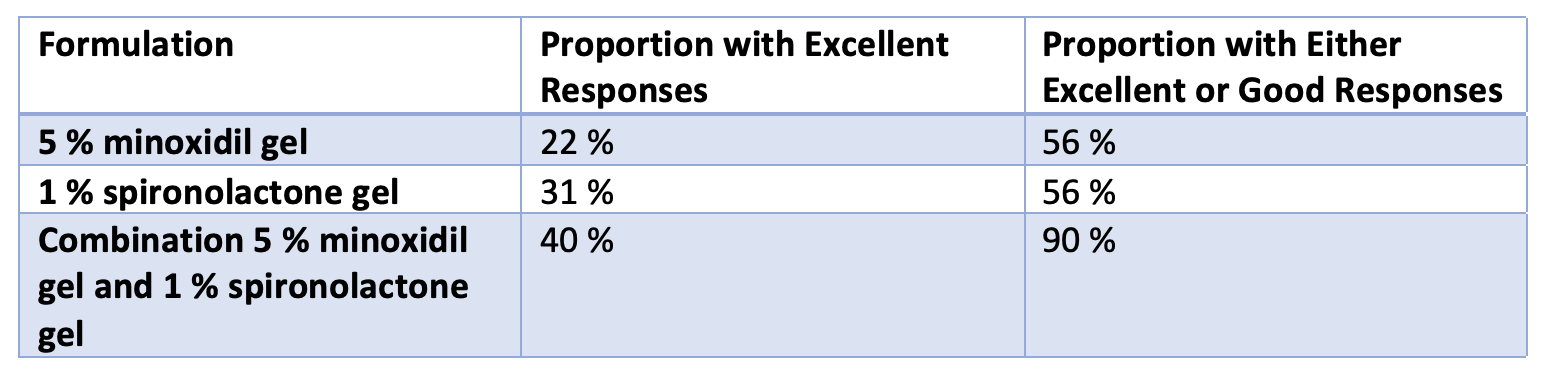
A new study suggested that topical spironolactone 1 % gel was helpful in over one half of users. In addition, the use of a combination product containing topical spironolactone and topical minoxidil produced good or excellent results in 90 % of users.
60 patients participated in the study - including 20 who received 5 % topical minoxidil alone, 20 who received 1 % spironolactone alone and 20 who received the combination of 5 % minoxidil/1 % spironolactone. 58 % of the participants were male and 42 % were female. The ages of the participants ranged from 18-45.
Results are shown in the table below. 56 % of those in the minoxidil alone or Spironolactone alone groups had either good or excellent results. In contrast, 90 % of those participants who were using the combined minoxidil-spironolactone product had good or excellent results. The main side effects of treatment was contact dermatitis which was experienced by 20 % of participants. There were no sexual side effects, no side effects related to libido and no gynecomastia reported.
Conclusion
This is a small study but an important in a few regards. First, it puts “topical” spironolactone on the map as a bonafide topical antiandrogen to consider in the management of androgenetic alopecia. The study needs repeating by other research groups to confirm or refute these observations. It’s not clear where topical spironolactone should be positioned among the main antiandrogens like topical finasteride but this study suggests if probably deserves a spot up there with the other antiandrogens.
I’m always a little concerned when I see such great numbers in clinical studies. Here, the response of patients using topical spironolactone alone was 31 % with excellent results and 56 % with either excellent or good response. I’m not sure even oral spironolactone can produce those kind of numbers! In other words, this study would suggest topical spironolactone is better than oral spironolactone where about 30 % get improvement. Similarly, this study suggests that 56 % of participants using 5 % minoxidil gel had excellent or good response. Again, I don’t think that 56 % of my patients with AGA who use topical minoxidil truly get this degree of benefit!!! So, the authors may have had a different scale for evaluating responses.
Nevertheless, I do feel this study is very helpful in its comparisons of topical minoxidil and topical spironolactone. Moreover, this study places topical spironolactone on the list as a topical antiandrogen that now deserves some serious study.
There were no serious safety concerns in this small study. Specifically there were no patients who experienced sexual side effects and no patients who had breast enlargement ( a potential side effects of oral spironolactone use). There was however a high incidence of contact dermatitis (20 %) with this formulation. That’s fairly high so additional studies may be needed to develop more acceptable formulations. 20% is an unacceptable range of contact dermatitis.
The final issue is the smell of this product. This was not discussed in the study. In case you did not know, topical spironolactone sometimes has a terrible smell in some formulations. The authors did not report anything about the tolerability of the 1 % spironolactone gel or the minoxidil/spironolactone combination.
It’s not so easy to just say to the local pharmacist “hey make me up some topical spironolactone!” One really needs to ask “hey can you make me up some topical spironolactone that does not smell! … do you know how to do that?”
All in all, a helpful study in my mind that lifts topical spironolactone up to sit amongst the other topical antiandrogens. It would be a mistake to think we’re done with understanding how best to use topical spironolactone. We need to repeat these studies and determine how best to formulate it in an acceptable topical formulation.
REFERENCE
Abdel-Raouf H et al. A novel topical combination of minoxidil and spironolactone for androgenetic alopecia: Clinical, histopathological, and physicochemical study. Dermatol Ther. 2021 Jan;34(1):e14678.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.