Upadacitinib in Alopecia Areata: Evidence Continues to Grow
The JAK1 Inhibitor Upadacitinib May be Helpful in Challenging Cases of Alopecia Areata
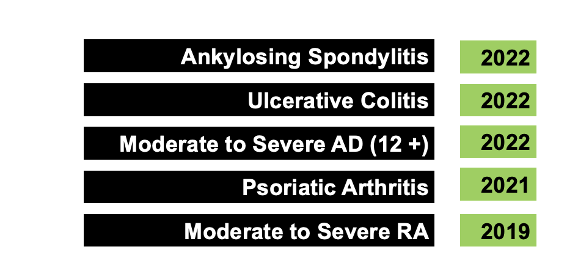
Upadacitinib is a JAK inhibitor and is an inhibitor of JAK 1. It was first approved by the FDA on August 16, 2019 for treatment of rheumatoid arthritis that doesn’t improve with use of TNF inhibitor medications. Since 2019, upadacitinib has received several FDA approvals for a variety of immune mediated conditions including psoriatic arthritis in 2021 and atopic dermatitis, ulcerative colitis and ankylosing spondylitis in 2022.
Upadacitinib in Alopecia Areata: A Closer Look at 4 Studies
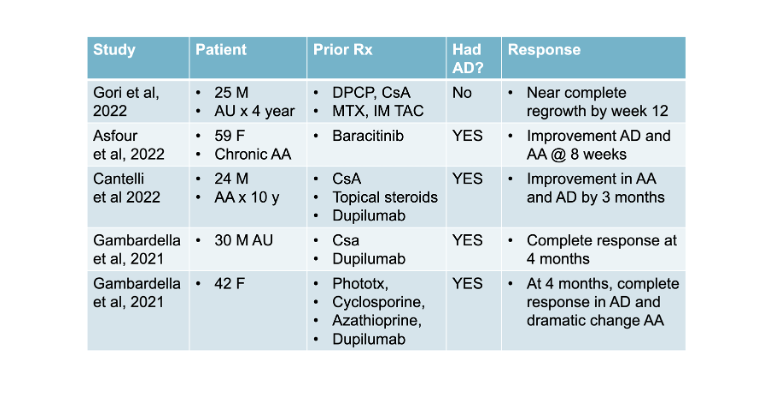
To date there have been 4 studies (including 5 patients) reporting a benefit of the drug upadacitinib in alopecia areata. 4 of the patients had atopic dermatitis and alopecia areata and received upadacitinib to treat atopic dermatitis as part of an FDA approved indication. One patient did not have atopic dermatitis and simply benefited from the drug.
Let’s take a look at these 4 studies.
Gambardella et al, 2021
In 2021, Gambardella and colleagues described 2 patients with alopecia areata and atopic dermatitis who responded to upadacitinib.
The first patient was a 30-year-old man with a severe atopic dermatitis and a five year history of alopecia universalis (SALT 100) resistant to conventional treatment. The patient was previously treated with cyclosporine for 6 months with only a partial response. In June of 2018, the patient began treatment with dupilumab the monoclonal antibody blocking interleukin 4 and interleukin 13. He had an excellent result for dermatitis; however, at week 28 into the treatment, he had a severe flare involving the face-neck area, which was not responsive to topical and systemic corticosteroids. He stopped Dupilumab and began 30 mg of upadacitinib. After 4 months of therapy, he had a complete remission of atopic dermatitis. In addition, the patient recovered all the scalp hair as well as eyelashes, eyebrows, and beard hair.
The second patient in the report was a 42-year-old woman with alopecia areata as well as severe atopic dermatitis, and asthma. Her prior atopic dermatitis treatments included UV-B narrowband phototherapy, cyclosporin, and azathioprine. She had a resistant patch of AA of the vertex (SALT score of 43). Dupilumab treatment was started, but at 18 weeks, there was no improvement of her dermatitis. Dupilumab was stopped at that time and a switch was made to upadacitinib 30 mg daily. After 4 months of therapy, a complete clinical response was obtained and she noted a dramatic patchy hair regrowth of the vertex
Gori et al, 2022
Gori et al reported the case of a 25-year-old man with a 4-year history of alopecia universalis (AU) who had a complete regrowth with upadacitinib at a dose of 30 mg daily. Of note here, he had previously failed treatment with topical diphencyprone and systemic agents (intramuscular triamcinolone, oral cyclosporine, and methotrexate). This patient did not have atopic dermatitis. Regrowth was near complete by week 12. There were no adverse events.
Asfour et al, 2022
Asfour and colleagues present the case of a 59-year-old woman presented with a 35-year history of relapsing–remitting alopecia areata with associated recession of the frontotemporal hairline and severe eyebrow loss. She has concurrent moderate to severe atopic dermatitis with significant hand involvement impacting her daily activities. The patient was initially treated with baricitinib, and her alopecia areata and atopic dermatitis improved somewhat. However, her bitemporal/preauricular patches and eyebrows did not show regrowth during her baricitinib course. Furthermore, she had to stop baricitinib after 6 weeks because of side effects including severe acne requiring systemic therapy, migraines, recurrent orolabial herpes simplex virus, and fatigue. She then started upadacitinib (dose not given). Within 4 weeks of her treatment being initiated, her eczema cleared. She also showed regrowth in her chronic AA patches near the ear within 4 weeks of starting upadacitinib and had a partial response in her eyebrows. She is tolerating the treatment well with no side effects at 8 weeks of follow up.
Cantelli et al, 2022
Authors from Italy presented the case of a 24-year-old patient with a history of atopic dermatitis since childhood and 10 years of alopecia areata. He had failed a variety of topical and systemic therapies, including corticosteroids and cyclosporine. He had severe AD [Eczema Area Severity Index (EASI): 45.1; and severe AA ( Severity of Alopecia Tool (SALT) score of 89.2). He was next started on dupilumab, the monoclonal antibody blocking interleukin 4 and interleukin 13. At the 4-month follow-up visit, he was noted to have some improvement in the body skin lesions. However, he showed red scaly lesions in the head and neck area, had associated itching and burning and was resistant to treatment with potent topical corticosteroids and antifungal medications. He also was diagnosed with a paradoxical “red face” due to dupilumab and dupilumab had to be stopped. After dupilumab the patient was prescribed upadacitinib at a dose of 30 mg/day. After 3 months of treatment , he had very significant hair growth and improvement in AD
Comment and Conclusion
These are interesting studies which suggest that upadacitinib may have a place on the list among the three currently used JAK inhibitors for alopecia areata: tofacitinib, ruxolitinib and baricitinib. Baricitinib of course stands by itself in those with severe alopecia areata as it is currently the only FDA approved drug.
However, upadacitinib has a unique benefit given its approval for atopic dermatitis in those 12 years of age and over. Nearly 40 % of patients with alopecia areata have atopic dermatitis so there are going to be a very large number of patients with refractory alopecia areata who also have refractory atopic dermatitis. These patients may be candidates for the anti IL-4/IL-13 drug dupilumab and they may be candidates for the JAK1 inhibitor upadacitinib.
REFERENCES
Gambardella A, Licata G, Calabrese G, et al. Dual Efficacy of Upadacitinib in 2 Patients With Concomitant Severe Atopic Dermatitis and Alopecia Areata. Dermatitis 2021; 32: e85–e86.
Asfour et al. Concurrent chronic alopecia areata and severe atopic dermatitis successfully treated with upadacitinib. Int J Dermatol. 2022 Jun 20.
Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022; 1: e15346
Gori N et al. Assessment of alopecia areata universalis successfully treated with upadacitinib. Int J Dermatol. 2022 Jul 2.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.