Hydroxychloroquine (Plaquenil) Use, Pregnancy and Scarring Alopecia: What should we advise our patients?
Studies in Scarring Alopecia are Non-Existent; Studies from Lupus Literature Suggest Generally Low Risk
Hydroxychloroquine (Plaquenil, etc) is frequently used for the management of scarring alopecia. A clinical scenario which is encountered from time to time is whether women diagnosed with scarring alopecia who use hydroxychloroquine to treat the scarring alopecia and who are considering pregnancy or who are newly pregnant need to stop their hydroxychloroquine therapy.
Today, we’ll take a careful look at this question.
Hydroxychloroquine is known to cross the placenta and inhibits cell division and DNA synthesis. Therefore, hydroxychloroquine could theoretically have effects on rapidly dividing embryonic cells.
A lot of the information we have comes from studies of women with the autoimmune disease systemic lupus erythematosus (“SLE”) who use hydroxychloroquine during pregnancy. In fact, immunosuppressive agents are commonly used in SLE during pregnancy, to ensure the optimum outcome for both mother and child. The decision to continue immunosuppressives for women with lupus is really important. Women with SLE who continue HCQ throughout pregnancy seem have lower disease activity and lower prednisolone doses at the end of pregnancy than those who stop earlier. In other words, it’s often the recommendation for women with lupus to use hydroxychloroquine rather than not use hydroxychlorqouine.
In various systematic reviews, use of hydroxychloroquine in pregnancy does not seem to be associated with any increased risk of prematurity, spontaneous abortion or fetal death.
Let’s take a look at some helpful meta-analyses.
STUDY 1: Sperber et al. 2009
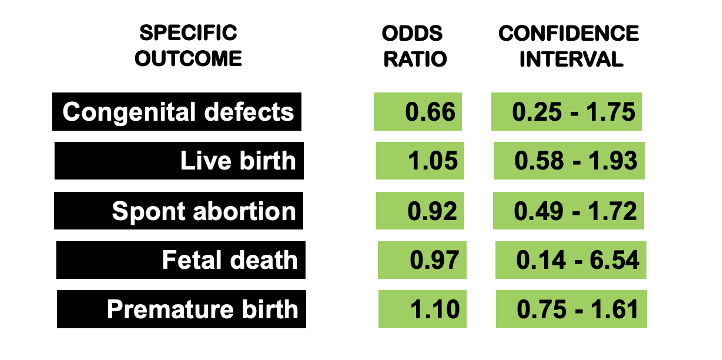
In 2009, Sperber et al set out to compare the incidence of congenital defects, spontaneous abortions, number of live births, fetal death and pre-maturity in women with autoimmune diseases taking HCQ during pregnancy. The authors identified all clinical trials from 1980 to 2007 addressing HCQ and pregnancy. The odds ratio (OR) of congenital defects in live births of women taking HCQ during pregnancy was 0.66, 95% confidence intervals (CI) 0.25, 1.75. The OR of a live birth for women taking HCQ during pregnancy was 1.05 (95% CI 0.58, 1.93). The OR of spontaneous abortion in women taking HCQ during pregnancy was 0.92 (95% CI 0.49, 1.72). The OR of fetal deaths in women taking HCQ during pregnancy was 0.97 (95% CI 0.14, 6.54). The OR of pre-mature birth defined as birth before 37 weeks in women taking HCQ during pregnancy was 1.10 (95% CI 0.75, 1.61).
STUDY 2: Kaplan et al, 2016
In 2016, Kaplan et al set out to determine whether gestational use of hydroxychloroquine (HCQ) for autoimmune disorders leads to an increase in the risk for adverse pregnancy outcomes. The study included studies up to 2014 inclusive (7 cohort studies and 1 RCT) and compared outcomes between 800 HCQ-exposed and 1130 control pregnancies. Overall, the meta-analysis reported no significant increases in rates of major congenital (OR 1.13, 95% confidence interval (CI) 0.59, 2.17), craniofacial (OR 0.62, 95% CI 0.13, 3.03), cardiovascular (OR 1.06, 95% CI 0.29, 3.86), genitourinary (OR 1.38, 95% CI 0.42, 4.53), nervous system malformations (OR 1.81, 95% CI 0.31, 10.52), stillbirth (OR 0.69, 95% CI 0.35, 1.34), low birth weight (OR 0.69, 95% CI 0.21, 2.27) or prematurity (OR 1.75, 95% CI 0.95, 3.24). The rate of spontaneous abortions, however, was found to be significantly increased in HCQ exposed pregnancies (OR 1.85, 95% CI 1.10, 3.13).
All in all, the authors concluded that prenatal exposure to HCQ for autoimmune diseases does not appear to increase the risk of adverse pregnancy outcomes except spontaneous abortion rate although the authors felt this may be associated with the underlying disease activity (bias by indication) and needs further investigation.
Clowse et al 2022
In 2022, Clowse et al performed a meta-analysis was to identify the potential benefits and harms of HCQ use within lupus pregnancies. The study included 668 pregnancies with 63% of patients using HCQ throughout pregnancy. Compared with pregnancies without HCQ, those with HCQ had lower odds of highly active lupus, but did not have different odds of fetal loss, preterm delivery or pre-eclampsia. HCQ reduced the odds of preterm delivery among women with low lupus activity, Overall, this study of lupus pregnancies demonstrates a decrease in lupus activity among woman who continue HCQ through pregnancy and no harm to pregnancy outcomes.
Hydroxychloroquine is Recommended During Pregnancy for Many Women with SLE
Readers should take note that for women with SLE, HCQ is recommended for all women during pregnancy as it reduces the risk of congenital heart block and could possibly also reduce the incidence of pre-eclampsia (a serious condition in pregnancy associated with high blood pressure and protein in the urine).
Furthermore, the American College of Rheumatology, American College of Obstetrics and Gynecology, British Rheumatology Society, and the European League Against Rheumatism have each published recommendations to guide the use of antirheumatic medications in pregnancy and lactation
Azathioprine, oral steroids, hydroxychloroquine, tacrolimus and sulfasalazine and TNF inhibitors are generally the only drugs considered reasonably safe. Methotrexate, Mycophenolate mofetil are not considered safe in pregnancy.
The Huybrechts et al, 2021 Study of 2045 Pregnancies
An important study to note with differing findings was a 2021 population-based cohort study by Huybrechts et al using claims data. The study included 2045 hydroxychloroquine-exposed pregnancies and 3,198,589 pregnancies not exposed to hydroxychloroquine. The authors reported a 1.26 adjusted relative risk of all major malformations among exposed pregnancies. No particular pattern of anomalies was found. The authors used advanced statistical methods to adjust for confounders.
In this particular study, it seemed that the risk for congenital abnormalities occurred mainly in women using higher doses of hydroxychloroquine. For example, although the overall adjusted relative risk was 1.26 (95% confidence interval, 1.04–1.54); it was 1.33 (95% confidence interval, 1.08–1.65) for a daily dose of ≥400 mg and 0.95 (95% confidence interval, 0.60–1.50) for a daily dose of <400 mg per day.
The authors continue to be of the view that most studies in the past that evaluated the teratogenicity of hydroxychloroquine were too small (36 -194 exposed) to be able to detect small to moderate risk increases. Huybrechts et al feel their study of 2045 patients has greater power to detect differences. In a 2021 report published in the American Journal of Obstetrics and Gyneologist titled “Hydroxychloroquine early in pregnancy and risk of birth defects: absence of evidence is not the same as evidence of absence” that authors write that they cannot dismiss signals of a potential small increased risk of congenital anomalies.
The study has fueled much discussion in the published medical literature. On one hand, it is clear for many women with lupus that not taking hydroxychloroquine is far riskier than taking it. Further more, some authors feel that the lack of any specific fetal abnormality in babies born to mothers using hydroxychloroquine points to a possible confounding effect. Still the data from the Huybrechts et al study will be with us a long time.
It does seem that if we are going to use hydroxychloroquine in our hair loss patients, low doses under 400 mg are going to be important and not using treatment if not necessarily will also be important. I am a big believer in using other treatment strategies if possible during pregnancy for my patients with scarring alopecia and limiting hydroxychloroquine to 200 mg three times per week starting in the second trimester. A prior article
Recent Studies
Good studies continue to come be published but continue to be fairly small. It not uncommon for studies of hydroxychloroquine to be a few dozen to a few hundred. studies of the size by Huybrechts et al are uncommon.
A 2021 study by Berard et al titled “Chloroquine and Hydroxychloroquine Use During Pregnancy and the Risk of Adverse Pregnancy Outcomes Using Real-World Evidence” found no clear increased risk of prematurity, LBW, or MCM. The study however had only 288 pregnancies.
A 2021 study by Howley titled “Maternal exposure to hydroxychloroquine and birth defects” did not find any clear evidence for a risk of specific birth defects. The study however had less than 20 pregnancies.
2021 study by Andersson titled “Fetal safety of chloroquine and hydroxychloroquine use during pregnancy: a nationwide cohort study” found no increased risk of major birth defects, preterm birth, or small for gestation age. The study however had only 303 patients exposed to hydroxychloroquine.
REFERENCES
Huybrechts K et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol 2021 Mar;224(3):290.e1-290.e22.
Michelle Petri. Pregnancy and Systemic Lupus Erythematosus. Best Pract Res Clin Obstet Gynaecol. 2020 Apr;64:24-30.
Talabi and Clowse. Antirheumatic medications in pregnancy and breastfeeding. Curr Opin Rheumatol 2020 May;32(3):238-246.
Krista F Huybrechts et al. Hydroxychloroquine early in pregnancy and risk of birth defects: absence of evidence is not the same as evidence of absence. Am J Obstet Gynecol. 2021 May;224(5):549-550.
Bonnie L Bermas and Christina Chambers. Hydroxychloroquine early in pregnancy and risk of birth defects: don't throw out the baby with the bathwater. Am J Obstet Gynecol . 2021 May;224(5):548-549.
Bérard et al. Chloroquine and Hydroxychloroquine Use During Pregnancy and the Risk of Adverse Pregnancy Outcomes Using Real-World Evidence. Front Pharmacol. 2021 Aug 2;12:722511.
Seo MR et al. Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus. 2019 May;28(6):722-730.
Howley et al. Maternal exposure to hydroxychloroquine and birth defects. Birth Defects Res. 2021 Oct 15;113(17):1245-1256.
Andersson et al. Fetal safety of chloroquine and hydroxychloroquine use during pregnancy: a nationwide cohort study. Rheumatology (Oxford). 2021 May 14;60(5):2317-2326.
Kaplan Y et al. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and meta-analysis. Br J Clin Pharmacol . 2016 May;81(5):835-48.
Clowse MEB et al. Hydroxychloroquine in the pregnancies of women with lupus: a meta-analysis of individual participant data. Lupus Sci Med. 2022 Mar;9(1):e000651.
Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J 2009;7:9.
Reynolds JA et al. Outcomes of children born to mothers with systemic lupus erythematosus exposed to hydroxychloroquine or azathioprine..Rheumatology (Oxford). 2022 Jun 29:keac372. doi: 10.1093/rheumatology/keac372. Online ahead of print.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.