New Study Shows Adolescents Respond Similarly to Ritlecitinib As Main Group.
New Study Shows Adolescents Respond to Ritlecitinib Similarly to Combined Adult and Pediatric Group
On June 23, 2023, Ritlecitinib (LITFULO) was FDA approved for treating advanced alopecia areata in patients 12 and older. We reviewed this medication and the exciting approval together back in June. To review a recording from our live public webinar, click the links below
The ALLEGRO 2b/3 Trials
It was the ALLEGRO 2b/3 trials that ultimately led to the FDA approval of ritlecitinib. These were studies where patients patients aged12 years or over with AA and ≥50% scalp hairloss received once-daily ritlecitinib 50 or 30 mg (±4-week 200-mg loading dose) or 10 mg or placebo for 24 weeks. There was a subsequent 24-week extension period whereby ritlecitinib groups continued their doses, and patients initially assigned to placebo switched to 200/50 or 50 mg daily.
The primary endpoint was the proportion of patients achieving a SALT score less than 20. However, a variety of clinician- and patient-reported hair regrowth outcomes and safety were assessed.
Overall about 23 % of patients receiving 50 mg achieved a SALT <20 and at week 24 and 43 % achieved a SALT <20 at week 48.
Ritlecitinib Adolescent Subgroup Analysis Shows Good Data for Adolescent Patients
A new study by Dr Hordinsky and colleagues sought to examine the ALLEGRO 2b/3 trial data as in pertains to adolescents.
In the ritlecitinib ALLEGRO 2b/43 trials, there were 718 total patients randomized to various groups. This included 105 adolescents (14.6%). 54 (51%) of these adolescent participants were female, mean age was 14.9 years, and 45 (43%) had AT/AU. Mean disease duration was 6.5 years.
How well did the 50 mg group do?
When I look at the trial data, there is a lot of data that can be seen. This is actually a pretty complex trial with many treatment groups. I like to focus on the 50 mg treatment group because that is the dose that was FDA approved. 30 mg was not FDA approved and a loading dose was not approved either.
SALT<20 at week 24
Across all participants receiving 30 mg or more, 17%–28% of adolescents achieved a Severity of Alopecia Tool (SALT) score≤20 (≤20% scalp without hair) by week 24 vs 0% for placebo. When one looks at the 50 mg group, this number was around 25 %.
SALT<20 at week 48
Across all participants receiving 30 mg or more, 25%–50% of adolescents achieved a Severity of Alopecia Tool (SALT) score≤20 (≤20% scalp without hair) by week 48 vs 0% for placebo. When one looks at the 50 mg group, this number was around 50%
Proportion of adolescents achieving SALT score less than 20. Results in adolescents are similar to data from the main ritlecitinib trial of all patients (adults and adolescents together)
SALT<10 at week 24
Across all participants receiving 30 mg or more, 6%–28% of adolescents achieved a Severity of Alopecia Tool (SALT) score≤10 (≤10% scalp without hair) by week 24 vs 0% for placebo. When one looks at the 50 mg group, this number was around 13 %.
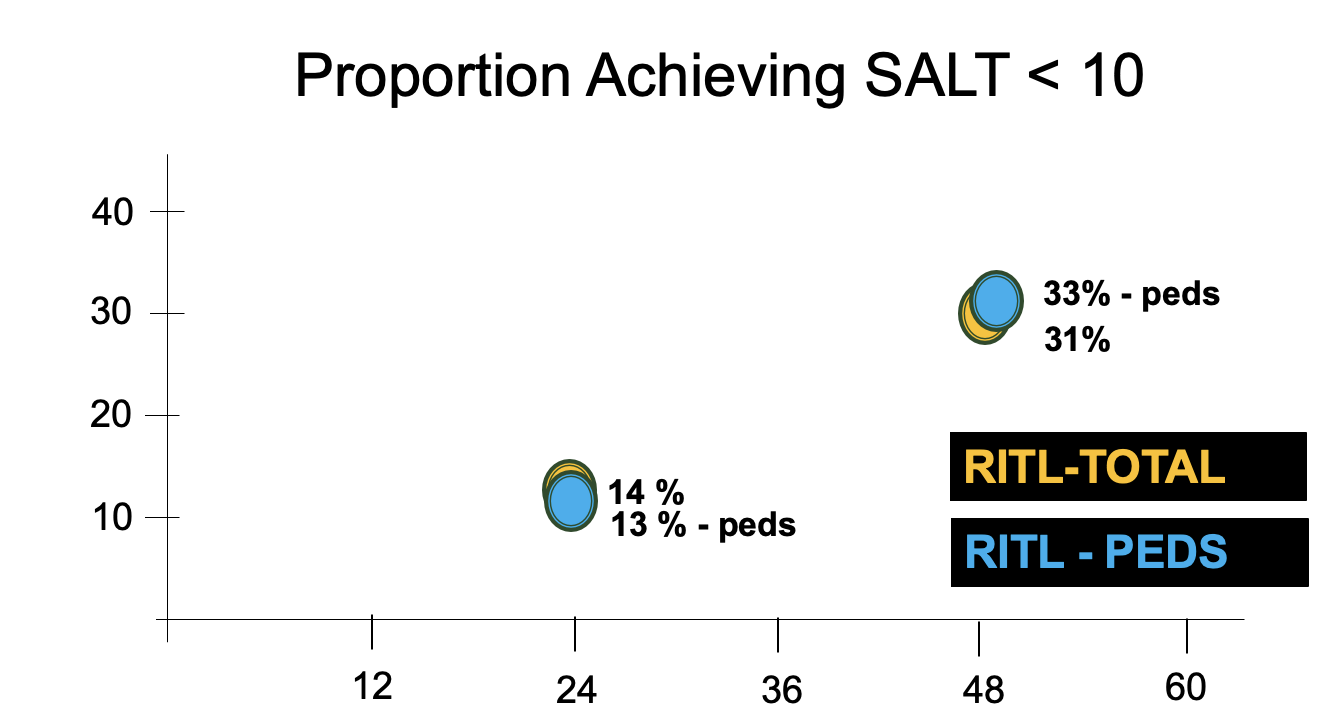
SALT<10 at week 48
Across all participants receiving 30 mg or more, 20%–50% of adolescents achieved a Severity of Alopecia Tool (SALT) score≤20 (≤20% scalp without hair) by week 48 vs 0% for placebo. When one looks at the 50 mg group, this number was around 33%
Proportion of adolescents achieving SALT score less than 10. Results in adolescents are similar to data from the main ritlecitinib trial of all patients (adults and adolescents together)
SIDE EFFECTS OF RITLECITINIB IN ADOLESCENTS
Ritlecitinib was fairly well tolerated. During the placebo-controlled period (first 24 weeks of the study), adverse events were reported in 65%–83% of adolescents across the ritlecitinib groups(with no clear dose effect) and 79% of adolescents in the placebo groups.
The most common side effects were headaches, acne and nasopharyngitis. 2 adolescents stopped the study. One in the 50 mg group stopped due to urticaria and 1 in the ritlecitinib 10 mg stopped due to eczema.
Across the entire study serious AEs were reported in 3 adolescents: one patient had appendicitis (200/30 mg group), one patient had suicidal behavior (10 mg group), and one patient had eczema (10 mg group). There were no deaths, malignancies, major adverse cardiovascular events, pulmonary embolisms, opportunistic infections, or herpes zoster infections were reported in this adolescent age group. There were no alterations in growth patterns.
Blood tests had slight changes. Creatinine kinases (CKs) bumped a bit in some participants; There was no rhabdomyolysis. Cholesterol levels also changed slightly but it was minor.
Conclusions
All in all it appears that ritlecitinib is reasonably well tolerated in the 12-17 age group. We have only 18 participants who received the 50 mg dose – i.e. the dose that is FDA approved so there is not all that much data to look at.
Nevertheless, it appears that improvements are very similar to what we see in the main ritlecitinib trial of all patients (adults and adolescents together). 25 % achieving SALT <20 at week 24 and about 50 % by week 48.
Side effects are mild in the pediatric group although there are fewer patients to collect data on. It will still be important to follow blood tests before and after starting as clearly some labs can change. Minor changes in CK and cholesterol were noted but appeared minor.
Given that this drug will be needed lifelong, it will be important to see what side effects take place over 5, 10 and 20 years or more of the drug and if results are maintained for many, many years or whether some patients lose effects and develop worsening hair loss again.
REFERENCE
Hordinsky M et al. Efficacy and safety of ritlecitinib in adolescents with alopecia areata: Results from the ALLEGRO phase 2b/3 randomized, double-blind, placebo-controlled trial. Pediatr Dermatol. 2023 Jul 17.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.