Hyaluronidase: A Potential New Treatment for Cutis Verticis Gyrata
Hyaluronidase Injections (150-200 IU) To Treat of Cutis Verticis Gyrata
A few recent studies support the use of an enzyme known an hyaluronidase for treating cutis verticis gyrata (CVG). CVG has been reviewed in the past. (see “Diagnosis and Treatment of Cutis Verticis Gyrata”)
Cutis verticis gyrata is a scalp condition associated with excessive growth of skin leading to furrows and ridges that very much resemble the folds and ridges of the brain.
Let’s first take a look at some of the key points about hyaluronic acid and hyaluronidase.
Hyaluronidase : General Points
Hyaluronic acid is an important part of the “extracellular matrix” (the material found in the skin between cells that hold everything together). One of the key responsibilities of hyaluronic acid is to keep the dermis hydrated.
Hyaluronidase (note the ending “ase”) is an enzymes that breaks down hyaluronic acid. Hyaluronidase is used in cosmetic dermatology to help quickly dissolve hyaluronic acid fillers. If hyaluronic acid fillers are placed into the skin into areas they shouldn’t have gone, hyaluronidase comes to the rescue to enzymatically break down the filler.
Hyaluronidase enzymes can come from many sources, but in the field of medicine, the most common sources is human recombinant hyaluronidase. This form of hyaluronidase has the lowest chance of allergic reactions compared to other forms (like bovine forms). Even though chance of allergy is fairly low, all physicians who use hyaluronidase in non emergent situations need to considering doing a small patch test on the forearm with 8-20 units and wait 30 minutes to see if any type of allergic reaction develops. If an allergy develops, one can’t proceed with using the hyaluronidase.
Physicians inject hyaluronidase by mixing (reconstituting) the hyaluronidase with 1 to 10 mL of bacteriostatic saline (salt water) or with other anesthetics (like lidocaine). The hyaluronidase is injected into the skin with tiny 30 G needles. Depending on the exact reason for using hyaluronidase, the area may be massaged to help. Patients are then observed for one hour. Less than 1 in 1000 patients are reported to develop “hives” (urticaria) or angioedema. Anaphylaxis is a rare complication with higher doses.
It’s important to be aware that some medications block the full effect of hyaluronidase. These medications are referred to as “antagonists.” For this reason, a full medication history is needed before injecting hyaluronidase. Certain anti-inflammatory drugs like the NSAIDs, some allergy medications (i.e. antihistamines and mast cell stabilizers), as well as some vitamins and supplements (Vitamin C and other anti-oxidants) potentially act as antagonists of hyaluronidase. Patients preparing for hyaluronidase injections are often advised to stop all non-essential medications and supplements for 7 days. Essential medications must continue. A full medical history is also important before injecting hyaluronidase. A history of allergy to bee or wasp stings or snake bits or insect reactions may present a relative contraindication to receiving hyaluronidase. Of course, a previous allergic reaction to use of hyaluronidase would also be a contraindication to performing the procedure again.
Treatment for CVG is optional for many patients. There are a large number of relatively asymptomatic patients and many patients with CVG do not even known they have the condition and many who do know do not proceed with treatment. However, those patients with significant deformity, odour, infections, pain, burning, may elect to undertake steps to improve the CVG and improve their quality of life. Treatments for CVG are mainly surgical for those who wish to consider treatment.
Hyaluronidase for CVG
Two new studies support the use of hyaluronidase for CVG.
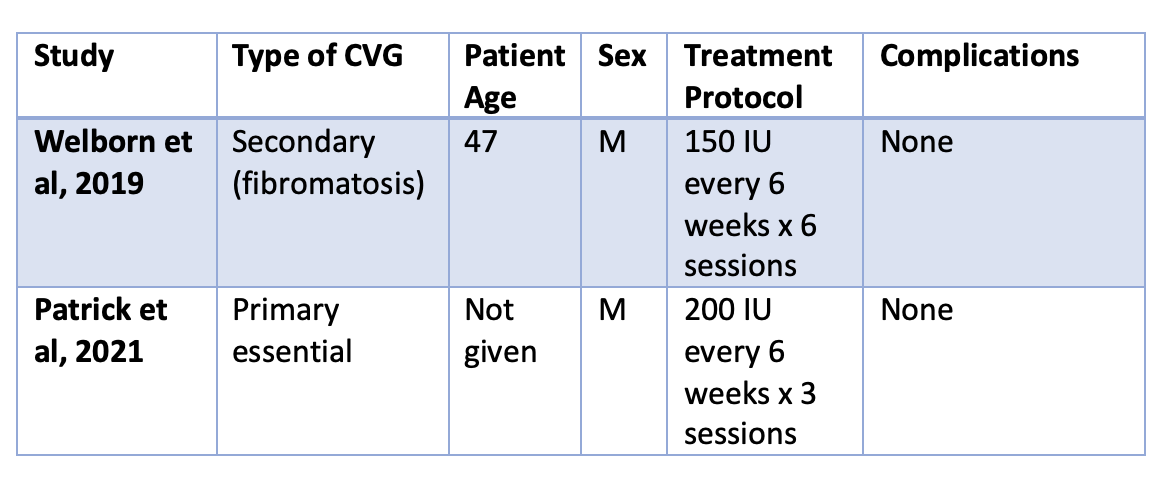
Study 1: Welborn et al, 2019
Welborn et al published a study in 2019 describing the use of 150 IU hyaluronidase every 6 weeks for 6 session in a 47 year old male patient with secondary CVG . The patient had a diagnosis of extra-abdominal fibromatosis. The paper and images can be downloaded here.
Study 2: Patrick et al, 2021
Patrick et al published a study in 2021 describing the use of 200 IU hyaluronidase every 6 weeks for three sessions in a male patient with primary essential CVG. There procedure was well tolerated the improvements were sustained after 1 year of follow up. There were no side effects. The authors do not describe the total volume of hyraluronidase used or whether lidocaine was used nor the source of the hyaluronic acid. The age of the patient was not given. The paper and images can be downloaded here.
Final Comment
These are two very exciting studies. Treatment of CVG is challenging and these two studies present new options that appear to have good safety and tolerability.
More studies are clearly going to be needed with regard to the long term effects and whether several hyaluronidase injections will create lasting effects or whether injections will need to be repeated.
Physicians interested in injecting hyaluronidase should have appropriate training in the use of hyaluronidase. Side effects and potential side effects of this procedure needs to be discussed with patients and potential contraindications reviewed. Obtaining full informed consent is necessary.
The amount of hyaluronidase injected in these studies for CVG is much higher than we inject for dissolving hyaluronic acid filler problems. For example, 3, 5, 10 or 20 IU might be needed to dissolve a filler. Here, 150-200 IU are used.
In conclusion, these are very promising studies and more research is needed to determine how best to treat CVG and how often repeat procedures should be done.
Reference
Cavallini et al. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers.
Aesthet Surg J. 2013 Nov 1; 33(8):1167-74.
Cohen BE et al. The use of hyaluronidase in cosmetic dermatology: a review of the literature. J Clin Investigat Dermatol. 2015;3(2):7.
Dunn AL et al. Review Hyaluronidase: a review of approved formulations, indications and off-label use in chronic pain management. Expert Opin Biol Ther. 2010 Jan; 10(1):127-31.
Patrick et al. Hyaloronidase as a Treatment for Cutis Verticis Gyrata. Plastic Surgery Case Studies 2021. Volume 7: 1-3
Welborn et al. Cutis Verticis Gyrata Improved with Injected Hyaluronidase Treatments. Dermatology Archives 2019.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.