Finasteride and Hydroxychloroquine in Frontal Fibrosing Alopecia (FFA): Are the really similarly effective?
Finasteride and Hydroxychloroquine in FFA
Download PDF Version of Article
A small study shows that 2.5 mg oral finasteride and “high dose” hydroxychloroquine (above 5 mg /kg) are fairly similar in their ability to halt disease activity in patients with frontal fibrosing alopecia. The typically recommended dose of hydroxychlorqouine was not assessed in this study (ie doses of 5 mg per kg or less).
Thirty-four patients were randomly assigned into two groups: one group received finasteride 2.5 mg once daily and one group received hydroxychloroquine 200 mg twice daily (400 mg total).
Both groups were also given the same topical treatment which included pimecrolimus (Elidel) twice daily for 5 days a week, mometasone once daily for 2 days a week, and minoxidil (5%) once daily. The use of sunscreens and moisturizing creams was allowed. Follow-up visits were scheduled after 0, 3, and 6 months of treatment.
The activity of the patient’s FFA was assessed using the 2018 Saceda-Corralo et al Frontal Fibrosing Alopecia Severity Score (FFASS) system as well as photography, and trichoscopy. The FFASS evaluates inflammation, redness, scaling and extension. It is described in the March 2018 issue of the Journal of the American Academy of Dermatology. FFASS was measured at baseline and at 3 and 6 months after treatment.
A dermatologist who was blind to the study and familiar with FFASS scored patients at each visit.
Two dermatologists who were blind to group allocation used the Global Aesthetic Improvement Scale (GAIS) a standardized 5-point relative scale range from worse (−1), no change (0), improved (1), much improved (2), and very much improved (3) to interpret the photography.
Results
Baseline characteristics of the two groups were similar including patient age, age of onset, premature menopause, facial papules, eyebrow and eyelash loss, and presence of rosacea.
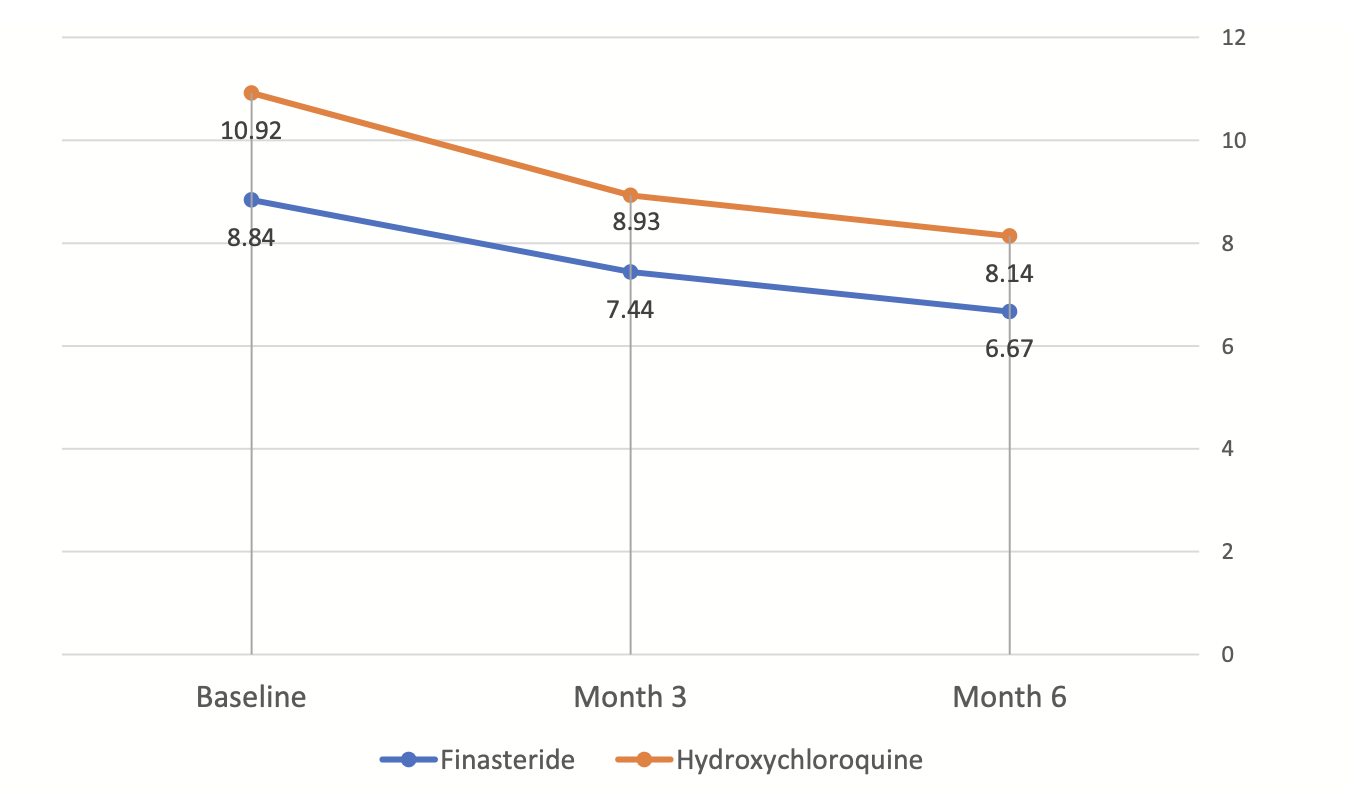
34 patients were enrolled in the study and 32 actually finished the study. This included 15 patients treated with hydroxychloroquine and 17 with finasteride, Both groups exhibited a significant decreasing trend in FFASS. Interestingly, there was no significant difference between the two groups during the study (p = 0.110)
Figure from: Saber M et al. Clinical effectiveness of finasteride versus hydroxychloroquine in the treatment of frontal fibrosing alopecia: A randomized controlled trial. J Cosmet Dermatol. 2023 Sep 10. Used here with creative commons license.
Trichoscopic signs improved in both groups with 6 months of treatment. This included perifollicular scaling, perifollicular erythema, milky-red area, and the total trichoscopic score.
Overall, there was no significant difference in trichoscopic signs and total trichoscopic score between the finasteride and hydroxychloroquine groups.
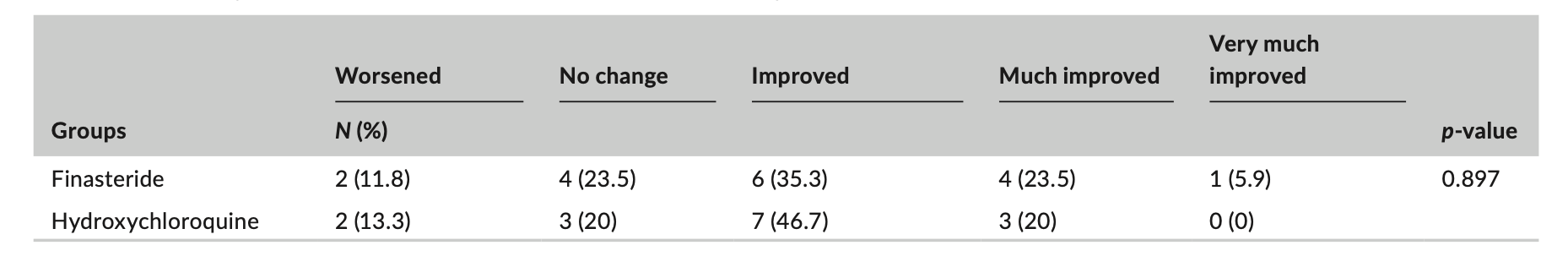
A proportion of patients in each group had improvement. In the finasteride group, 6 (35.3%) patients showed no change or worsening of the disease, and 11 (64.7%) patients showed mild to very significant improvement. In the hydroxychloroquine group, five patients (33.3%) exhibited no change or deterioration of the disease while 10 patients (66%) demonstrated improvement ranging from mild to significant. However, statistical analyses showed that there was no significant difference between the two groups.
Table from: Saber M et al. Clinical effectiveness of finasteride versus hydroxychloroquine in the treatment of frontal fibrosing alopecia: A randomized controlled trial. J Cosmet Dermatol. 2023 Sep 10. Used here with creative commons license.
In terms of side effects, finasteride and hydroxychloroquine were both were well- tolerated treatments for patients with FFA.
COMMENTS
I congratulate the authors for performing an RCT. These types of studies are so much needed in our field.
This data here shows that finasteride and “high dose” hydroxychloroquine can both help patients with FFA.
I’m not sure that we can truly conclude that finasteride and regular dose (typical dose) hydroxychloroquine are similarly effective. The dose of hydroxychloroquine in this study was likely too high so many patients probably received a dose that was not typical of what we would normally prescribe.
With an average height of women in Iran of 5 ft 4 and an obesity estimate of 30 %, it’s likely that 70% or more of women in this study received a dose of hydroxychloroquine that was above the recommended 5 mg/kg supported by the recent American Academy of Ophthalmology guidelines.
So all that we can say here is that finasteride 2.5 mg and high dose hydroxychloroquine are likely similar in effectiveness.
We don’t really know if 2.5 mg finasteride and the recommended dose of hydroxychloroquine will be similar in effectiveness. Possibly not.
In my clinic I don’t view hydroxychloroquine as quite the same effectiveness as finasteride or dutasteride. Certainly, this data in the RCT has caused me to question my view. But I’m not convinced with this data that we can really say they are the same. The doses of hydroxychloroquine here are too high.
I agree with the authors that hydroxychloroquine can be a really good option women of reproductive age, including those who intend to become pregnant or have a personal or family history of breast cancer. In can also be a good option for those who experience a mood disturbance with finasteride or those experiencing decreased libido on finasteride.
For now, I’m still keeping hydroxychloroquine as a second line agent in FFA. I use it alot and sometimes even combine it with finasteride or dutasteride !
REFERENCE
Saber M et al. Clinical effectiveness of finasteride versus hydroxychloroquine in the treatment of frontal fibrosing alopecia: A randomized controlled trial. J Cosmet Dermatol. 2023 Sep 10.
Saceda-Corralo D et al. “Development and validation of the Frontal Fibrosing Alopecia Severity Score,” Journal of the American Academy of Dermatology. March 2018. DOI: https://doi.org/10.1016/j.jaad.2017.09.034
Melles RB et al. Hydroxychloroquine Dose and Risk for Incident Retinopathy : A Cohort Study. Ann Intern Med. 2023 Jan 17.
Melles & Marmor. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. . JAMA Ophthalmol. 2014 Dec;132(12):1453-60.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.