Another Study Pointing to Safety of Hydroxychloroquine During Pregnancy
New Study Indicates Hydroxychloroquine Does not Increase the Risk of Congenital Malformations
From time to time, we review the safety data on hydroxychloroquine in pregnancy. It’s an important subject for hair specialists to know given that some women of child bearing age with scarring alopecia do use hydroxychloroqouine. Do patients need to stop? Can they start the medication if needed during pregnancy?
We’ve discussed the safety of hydroxychloroquine in pregnancy in prior articles including several meta-analyses that suggest that hydroxychloroquine does not increase the risk of fetal abnormalities,
Today, we’ll discuss another new study highlighting what appears to be good safety of hydroxychloroquine in both pregnancy and breastfeeding.
Reynolds et al, 2022
A recent study set out to retrospectively determine the outcomes of children born to mothers with SLE exposed to HCQ or AZA during pregnancy and breast-feeding.
The study population included women diagnosed with lupus attending UK specialist lupus clinics with children under 17 years of age. Children born to mothers diagnosed with SLE were recruited to this retrospective study. The authors analysed 284 live births of 199 mothers from 10 UK centres. The first pregnancies of 73.9% of mothers (147/199) were captured in the study.
Prednisolone was used in 59.1 % of pregnancies (i.e. 167/283), and HCQ was used in 52.7 % of pregnancies (i.e. 149/283). AZA was used in 30.4 % of pregnancies (ie 86/283). In 38/283 pregnancies, all 3 medications were prescribed. The median follow-up of children was 3.23 years.
60.4% of children were exposed to HCQ and 31.1% of children were exposed to azathioprine. There were no significant differences in the frequency of congenital malformations or intrauterine growth restriction between children exposed or not to HCQ or AZA. In adjusted models, exposure to AZA was associated with increased reports of childhood infection requiring hospital management [odds ratio 2.283 (1.003, 5.198), P < 0.049].
Discussion and Comments
I liked this study. I noted that this is one of the largest studies examining outcomes in children born to mothers with SLE beyond the early post-natal period.
This particular study again did not find associations between HCQ and AZA exposure during pregnancy and increased risks of pre-term birth, IUGR or congenital abnormality. Other outcomes, including pre- eclampsia, neonatal lupus rash and congenital heart block did not differ in frequency between groups exposed to HCQ or AZA. All in all, the authors concluded that there were no significant negative outcomes in children exposed to HCQ in pregnancy. They felt that HCQ is compatible with pregnancy and breastfeeding. AZA was not associated with adverse events in either the mother or foetus. However, AZA use may be associated with increased reporting of childhood infection and this unto itself warrants further study.
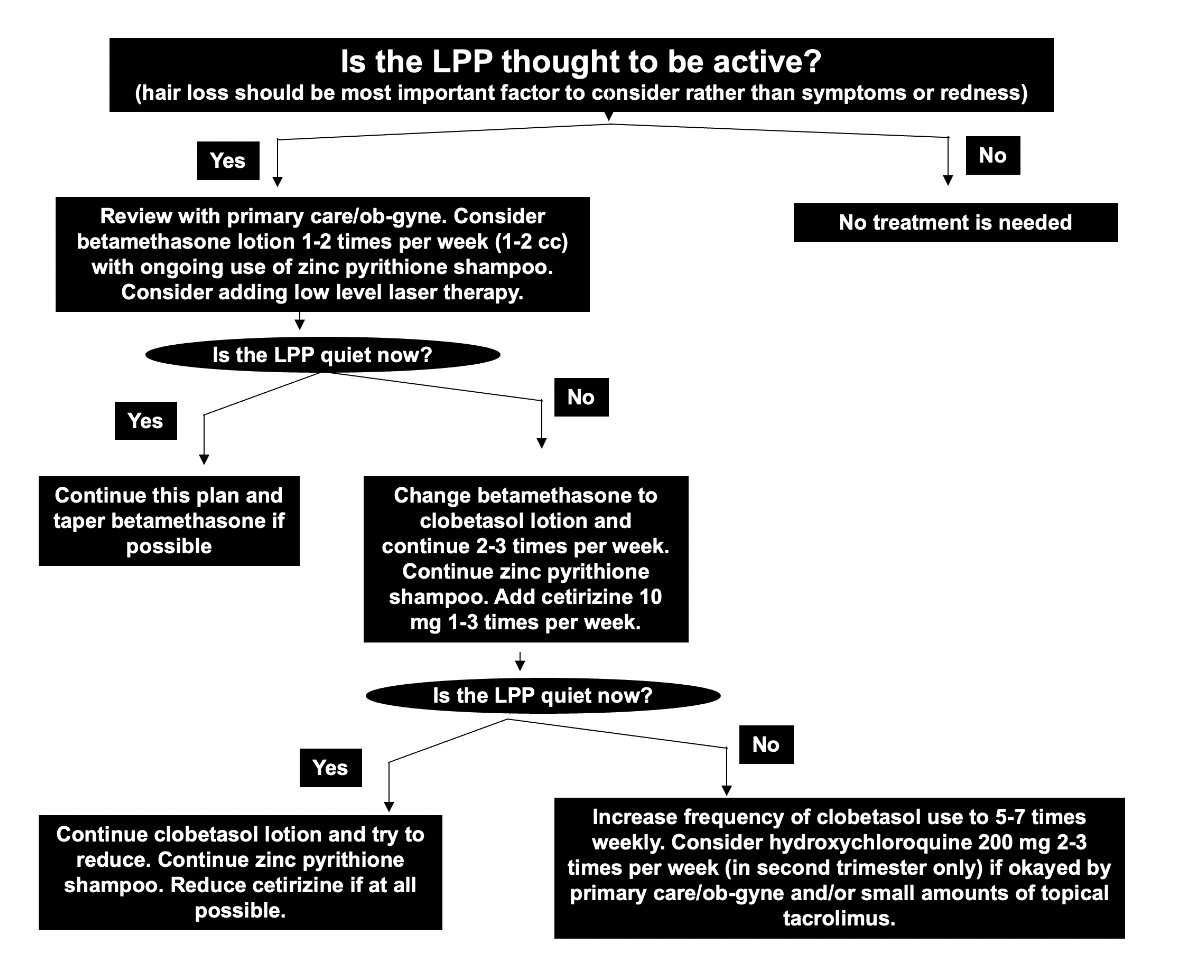
It’s helpful to review prior discussions of hydroxychloroquine in pregnancy as well as my views on the management of lichen planopilaris and scarring alopecia in pregnancy in general. I have provided care for many patients with scarring alopecia and many patients can be managed during pregnancy with periodic use of mild topical steroids and antihistamines and low level laser and shampoos. If necessary, hydroxyhcloroquine and stronger topical steroids like clobetasol can be started in the second trimester but most patients do not require it in my experience. Small amounts of tacrolimus ointment (Protopic ointment) seem safe as well.
REFERENCE
Reynolds JA et al. Outcomes of children born to mothers with systemic lupus erythematosus exposed to hydroxychloroquine or azathioprine. Rheumatology (Oxford). 2022 Jun 29:
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.