Meta-Analysis Highlights Good Short Term Safety of JAK Inhibitors for Dermatologic Issues.
No Increased Risk in Blood Clots, Cardiac Events or Death Identified
Janus kinase inhibitors have entered onto the scene as helpful treatments for many diseases in dermatology including alopecia areata, atopic dermatitis and vitiligo. As we’ve talked about many times over the years, there remains a “boxed warning” from the FDA on JAK inhibitors warning the public and physicians about the possible increased risk of major adverse cardiovascular events (MACE), venous thromboembolism (VTE), serious infections, malignant neoplasm, and death.
This boxed warning comes about from past studies like the ORAL Surveillance Study which showed that patients with rheumatoid arthritis who were 50 years of age and older and who had at least 1 cardiovascular risk had increased risk for major adverse cardiac events, infections, cancer venous thromboembolism and death from using the JAK inhibitor tofacitinib. We have reviewed the ORAL Surveillance Trial in the past:
As JAK inhibitors continue to be studied and continue to be approved for a variety of dermatologic conditions, the question that dermatologists ask themselves over and over is “do these ORAL Surveillance trial results really and truly apply to patients with skin diseases like it does to patients with rheumatologic diseases? Does a patient with alopecia areata or vitiligo or atopic dermatitis also have an increased risks for things like blood clots and heart disease?
Ingrassia JP et al 2023
Authors of a new systematic review and meta-analysis set out to determine the risk of all-cause mortality, MACE, and VTE in patients specifically with dermatologic conditions who used JAK inhibitors.
The authors included dermatology patients who were participating in phase 3 randomized clinical trials of an approved JAK inhibitor for the treatment of an immune mediated inflammatory skin disease - provided the trial also had a placebo/active comparator group. Studies without a comparison group, case reports, observational studies, and review articles were excluded.
The primary outcome was major adverse cardiac events (MACE) and all-cause mortality, and VTE.
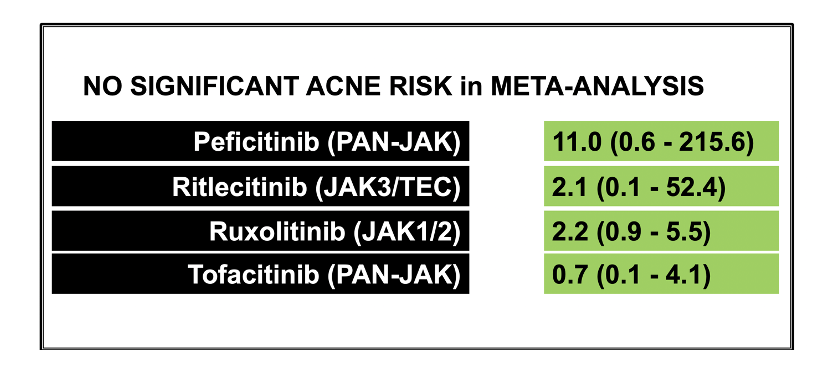
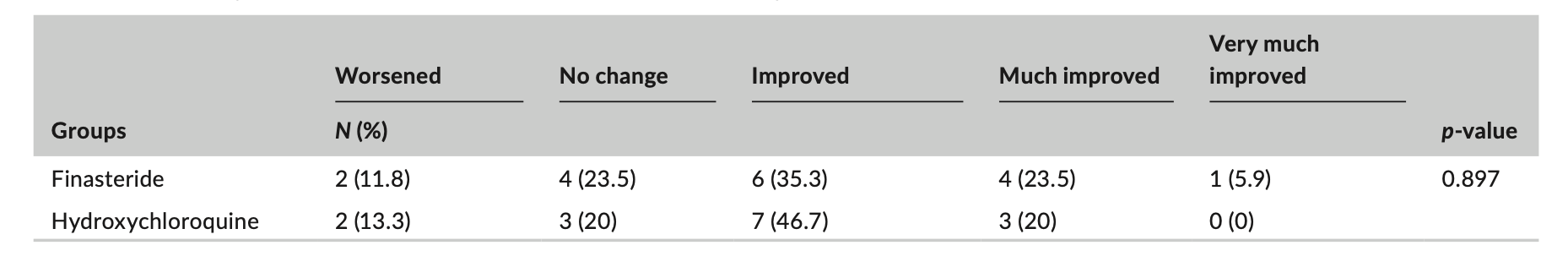
The authors included in their meta-analysis a total of 35 RCTs (8 with baricitinib, 6 with abrocitinib, 2 delgocitinib cream, 4 ruxolitinib cream, 7 with tofacitinib, 1 with ritlecitinib, and 7 with upadacitinib). The analysis included 20 651 patients (mean age, 38.5 years; male, 54%). The mean follow up time was quite short age 4.9 months.
The trials enrolled a total of 20 651 patients. This included 13 597 patients who were randomized to a JAK inhibitors and 7054 to placebo/active comparators. Dermatologic diseases included atopic dermatitis (21 trials), alopecia areata (3 trials), psoriasis (including psoriatic arthritis) (9 trials), and vitiligo (2 trials).
All in all, the authors’ findings did not show a significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (OR, 0.83; 95% CI, 0.44-1.57) or VTE (OR, 0.52; 95% CI, 0.26-1.04).
Comments
This is a helpful study for all of us in dermatology who use JAK inhibitors to treatment various autoimmune skin diseases.
What I particularly liked in this paper was the honest and straightforward discussion. The authors point out that there are many differences between the studies included in this meta-analysis and the ORAL SURVEILLANCE TRIAL.
The authors point out to readers that
a) The patients in this study with dermatologic conditions were younger (mean age, 38.5 years) than the patients with RA (mean age, 61 years). This fact alone could explain the low overall incidence of MACE, all-cause mortality, and VTE among patients in this meta-analysis (of the dermatology clinical trials).
b) Patients in the ORAL Surveillance had higher cardiovascular risk than the patients in the meta-analysis because the ORAL surveillance trial only enrolled patients with RA with at least 1 cardiovascular risk factor.
c) Patients in the ORAL SURVEILLANCE TRIALS received methotrexate and some were also receiving systemic corticosteroids. This is very different than patients included in dermatology clinical trials, who were receiving JAK inhibitor monotherapy.
d) The dermatology meta-analysis here included trials with short duration (4.9 months). The ORAL SURVEILLANCE STUDY followed patients for 4 years whereas this meta-analysis followed patients for a far shorter period
e) In addition, most of the new JAK inhibitors used in this particular meta-analysis were JAK 1/2 inhibitors rather than JAK 1-3 inhibitors like (tofacitinib)
f) Finally, the authors point out to readers that in this study, they did not focus on all the boxed warnings but rather 3 of the 5 possible boxed warning: MACE, VTE, and death. The authors did not examined infections and cancer in this study ..
For now, what we can say is that in these 35 trials of fairly short duration of follow up, there is no major signal for venous thromboembolism and major adverse cardiac events. This is good news so far for all of us who use these medications!
REFERENCE
Ingrassia JP et al. Cardiovascular and Venous Thromboembolic Risk With JAK Inhibitors in Immune-Mediated Inflammatory Skin Diseases A Systematic Review and Meta-Analysis. JAMA Dermatol. 2023 Nov 1:e234090.
Ytterberg SR et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022 Jan 27;386(4):316-326. doi: 10.1056/NEJMoa2109927.PMID: 35081280 Clinical Trial.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.